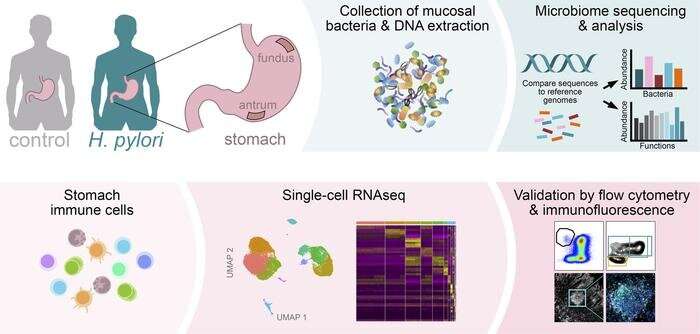
The first detailed description of the microbiota and immune cells among asymptomatic Helicobacter pylori-infected individuals has been published by researchers at Karolinska Institutet. The results of the study will be instrumental to understanding the complex microbiome and immunity network and provide new insights for asymptomatic Helicobacter pylori infection.
Although H. pylori chronically infects half of the world’s population, only a small segment of infected individuals develops symptoms—the majority are asymptomatic. Research has shown that these people might have a higher risk for developing cancer. But very little information is known about the microenvironment in the asymptomatic stomach.
In the study, published in JCI Insight, the research team conducted a detailed gastric microbiome and immune cell composition analysis and identified potential bacterial functions and effects on the host immunity in H. pylori-infected asymptomatic individuals.
“Using single cell RNA sequencing and flow cytometry, we found H. pylori-infected individuals were characterized by a higher proportion of activated immune cells, which are known to participate in adaptive immune responses involving antibody production and anti-tumor responses. Thus, the microbiota and immune cell landscape from H. pylori-infected asymptomatic individuals are heavily altered and may have long-term effects,” says Juan Du, docent at the Department of Microbiology, Tumor and Cell Biology, Karolinska Institutet, and one of the corresponding authors of the study.
Valuable resource for gastric diseases research
“Our study used the most up-to-date methods, and showed the detailed gastric microenvironment, which undergoes deep microbial and immunological changes during chronic H. pylori infection. The whole genome sequencing of gastric mucosa bolsters the knowledge of stomach physiology, with respect to the gastric microbiome and microbiota function.
“We also present a comprehensive immune cellular landscape of the human stomach, which will be a valuable resource to interrogate pathology of gastric diseases, both for research and clinical setting, to follow H. pylori infected asymptomatic individuals,” says Eduardo Villablanca, Principal Researcher at the Department of Medicine, Karolinska Institutet, and one of the corresponding authors of the study.
The next stage of research
“This setting gave us the unique chance to analyze gastric tissue from H. pylori-infected individuals without clinical manifestations of gastritis. If the data could be validated with a larger cohort of other individuals than obese people, this will make our findings more solid. A complete immune cells survey for scRNA-seq analysis will provide the missing information from this study.
“Further mechanism studies are also needed to prove if H. pylori infection is the cause of all the changes observed in the gastric microbiome and immune cells. The results of the study will be instrumental to understanding the complex microbiome and immunity network and provide new insights for asymptomatic Helicobacter pylori infection,” says Juan Du.
Karolinska Institutet

