What happens during an epileptic seizure? A recent study suggests that seizures occur after certain defense cells in the brain break down.
Epileptic seizures can happen to anyone. In Norway alone, 35 000 people live with an epilepsy diagnosis. Epilepsy can be due to various injuries, diseases or malformations in the brain.
“Seizures can arise abruptly and apparently from nowhere. The fear of new seizures can itself create limitations in daily life,” says Sverre Myren-Svelstad, a doctor specializing in neurology and a Ph.D. candidate at NTNU’s Department of Neuromedicine and Movement Science.
But how does an epileptic seizure occur?
New results show that a failure or breakdown of the so-called glial cells in the brain underlies the triggering of an epileptic seizure. These results have now been published in Nature Communications.
A failure or breakdown of the so-called glial cells in the brain underlies the triggering of an epileptic seizure. Photo: Shutterstock, NTB Scanpix
The answers are not all in yet, but the Yaksi group at NTNU has discovered several of them in collaboration with the Department of Neuromedicine and Movement Science, the Department of Clinical and Molecular Medicine at NTNU and NERF (Neuro Electronics Research Flanders) at VIB, IMEC and KU Leuven in Belgium.
Studying epileptic seizures in zebrafish
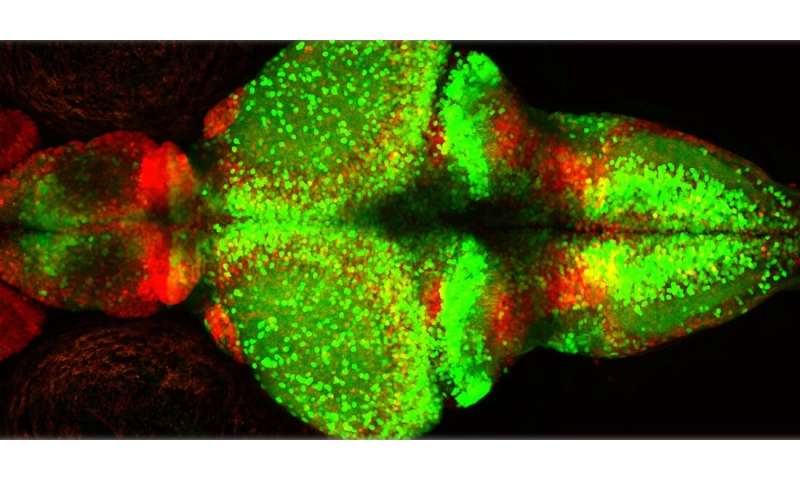
The research group studied epileptic seizures in zebrafish (Danio rerio). One advantage of these fish is that they are transparent, and activity in their nervous system is easier to observe than in many other species.
Nerve cells (or neurons) and glial cells are part of the networks that transmit signals in the brain.
- Neurons are primarily involved in transmitting signals.
- Glial cells are found in several variants. Their main functions include maintaining a balanced environment and providing support for the neurons, assisting the immune system and increasing the speed of neural signaling.
The research group found typical patterns just before and during the epileptic seizures:
- In the beginning phase, just before an epileptic seizure, nerve cells were abnormally active but only in spatially localized area of the brain. Instead, glial cells showed large bursts of synchronous activity, widely dispersed across the brain. In zebrafish, this activity in the glial cells looked like lightning shooting through the brain.
- During the actual seizure, the neuronal activity increased abruptly. The functional connections between the nerve cells and glial cells, and between different glial cells, became very vigorous. When this happened, generalized seizure spread like a storm of electrical activity across the entire brain.
- During the generalized seizure, the research group also noted a strong increase in the level of glutamate. Glutamate is a neurotransmitter, a chemical compound that transmits signals between cells in the network.
Glial defenses collapse during epileptic seizures
The main theory is that the continuous accumulation of local neural activity is what unleashes the subsequent cascade of events. In human patients, this may be initiated due to a disease state, injury or malformation in the brain.
The strong firing by the glial cells before the seizure probably represents the protective function of glial, as glial activity bursts before the seizures corresponds to transient silencing of neurons during pre-seizure state.
“The hyperactivity of the glial cells before a seizure is most likely a defense mechanism. The glial cells are known to absorb the excess glutamate secreted during the increased activity of the nerve cells,” says Nathalie Jurisch-Yaksi, group leader at NTNU’s Department of Clinical and Molecular Medicine and a partner in this project.
The glial cells thus temporarily prevent the nerve cells’ overproduction of excitatory neurotransmitters from leading to a seizure. But the glial cells only sustain this protective function for a transient period, until it becomes too much neural activity to handle.
“We believe that at some point the defenses break down. The glial cells become unable to absorb the high levels of the glutamate neurotransmitter. When it gets to be too much for them, the glial cells simultaneously release all the glutamate they’ve already absorbed. Suddenly the brain is hit with a very high level of glutamate. We believe this excessive release of glutamate by the glial cells leads to a generalized seizure spreading across the brain,” says Carmen Diaz Verdugo, a Ph.D. candidate.
Hence, a massive increase in glutamate overwhelms the brain, leading to a seizure.
Glia-neuron interactions change during seizures
The findings may also indicate that epilepsy may occur not only due to anomalies in neurons, but also can be related to the pathological conditions in glial cells, and abnormal interactions between glia cells and neurons.
Previous studies of patients and in animal models have shown that the properties of the glial cells change after repeated epileptic seizures. But less was known about how the function of the glial cells changes before and during the seizures.
“Our results provide a direct evidence that the interactions between glial cells and neurons change during the transition from a pre-seizure state to a generalized seizure. It will be interesting to see if this phenomenon is generalizable across different types of epilepsies,” says Professor Emre Yaksi.
May inspire new therapies
In recent decades, a number of new epilepsy drugs have been developed, but a third of patients still do not have good control over their seizures. One reason may be that the current anti-epileptic drugs mostly target the neurons, while the glial cells, which constitute ~80% of the cells in the brain, have been overlooked.
Given all this, gaining new knowledge about the role of glial cells in epilepsy, on the long term, could inspire novel therapies for patients suffering from seizures.
It is already known that some diseases associated with damage to the glial cells may increase the risk of epileptic seizures. Examples of such diseases are gliomas (brain tumors arising from glial cells) and multiple sclerosis. Moreover, damage to the glial cells is also observed in patients with Parkinson’s disease and Alzheimer’s disease, for instance.
“Now we’re working further to investigate whether we can recognize any of the mechanisms that we identified in our current study, in our ongoing collaboration with clinicians of St. Olav’s Hospital investigating genetic mutations causing epilepsy in patients. On the long term, training young clinicians in basic research is a major step-forward needed for translating fundamental discoveries to potential therapies in the clinic. We thank Helse-Midt Norge, St. Olav’s Hospital and Faculty of Medicine and Health Sciences at NTNU for their continuing generous support on our long-term vision,” says Yaksi.
Source: Norwegian University of Science and Technology

