A study led by researchers at Washington State University has uncovered a potential new treatment approach for diseases associated with inflammation, including sepsis, stroke, rheumatoid arthritis, acute lung injury, and atherosclerosis.
Published in the open-access journal Science Advances, their paper describes a novel, patent-pending technology that uses nanosized particles to transport cell-killing drugs directly to activated neutrophils, the cells that drive the exaggerated immune response involved in inflammatory diseases. They also demonstrated the technology’s feasibility at selectively killing activated neutrophils without harming other cell types or compromising the immune system.
“Scientists have started realizing that neutrophils—which were always seen as the ‘good guys’ for the key role they play in our immune system—are actually also contributing to the pathology of all kinds of diseases,” said the study’s senior author Zhenjia Wang, an associate professor in the WSU College of Pharmacy and Pharmaceutical Sciences.
You might think of them as beneficial cells that have gone rogue. Neutrophils, which make up as much as 70 percent of the body’s white blood cells, are the immune system’s first line of defense. Produced in bone marrow, they quietly patrol the blood stream, looking for viruses, bacteria, and other invading pathogens to fend off. They normally circulate through the blood for 8 to 20 hours before returning to the bone marrow to die as part of a natural process known as apoptosis—or programmed cell death—which helps keep the immune system in balance.
However, inflammation caused by pathogens or damaged tissue can activate neutrophils and keep them alive long beyond their normal lifespan. This increases neutrophil numbers in the blood and allows them to invade and accumulate in healthy tissue, resulting in damage that can harm organs and lead to death.
“Neutrophils don’t know who the enemies are,” Wang said. “They just attack, releasing all kinds of harmful proteins in the blood stream. They will kill bacteria, but they will also kill healthy tissue in the body at the same time.”

Nanoparticle design targets inflammatory neutrophils
Wang said previously studied approaches to target these activated, or inflammatory, neutrophils had a significant flaw: they not only killed off the harmful inflammatory neutrophils, but also the beneficial resting neutrophils in the bone marrow. This compromises the immune system and increases the chance of life-threatening, secondary infections.
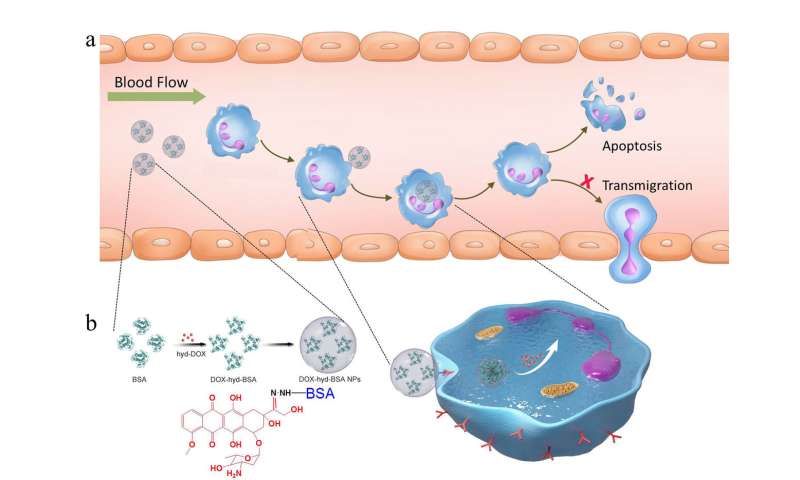
To address that issue, Wang and his research team created nanoparticles that are capable of carrying molecules of doxorubicin—a commonly used chemotherapy drug—into inflammatory neutrophils and release their drug load once inside.
They created these nanoparticles from albumin, a protein that naturally occurs in the blood stream. The technology relies on their discovery that Fc-gamma receptors—a specific type of receptor cells that are found on the surface of all neutrophils—are activated in inflammatory neutrophils, but not in resting neutrophils. Thus, the nanoparticles will only bind to—and kill—inflammatory neutrophils, leaving resting neutrophils unharmed.
To ensure that the drug doesn’t get released before it reaches the inflammatory neutrophils, the researchers designed the bond between the nanoparticle and the drug molecules to be sensitive to acid. Blood is slightly alkaline, so this design allows the nanoparticles to travel through the blood stream intact. Once the nanoparticles reach the neutrophils’ acidic interior, the bond between the nanoparticle and the drug molecule is cleaved and the drug is released.
Findings confirm feasibility
To test the feasibility of their technology, Wang’s team conducted studies that used rodent models of two inflammatory conditions: sepsis—a life-threatening condition caused by widespread inflammation in the body and the cause of more than a third of all hospital deaths—and ischemic stroke, which is caused by a blood clot that obstructs blood and oxygen flow to the brain and triggers a harmful inflammatory response when blood flow is restored. Findings from their study suggest that the nanoparticles could be successfully used to increase survival in sepsis and minimize neurological damage from stroke.
“Our experiment found that our doxorubicin albumin nanoparticles can decrease the lifespan of harmful neutrophils in the blood stream,” Wang said. “More importantly, we also found that our nanoparticles don’t inhibit the neutrophils’ function in the bone marrow.”
Wang and his team plan to conduct additional research to delve deeper into how their technology works on a molecular basis and to further optimize it. The next step would be to test the technology in human clinical trials before it could be developed into a commercially available treatment strategy that could benefit patients.
Washington State University

