New insight on how a type of cell facilitates the spread of HIV-1 has been published today in the open-access journal eLife.
The findings in mice suggest that subcapsular sinus macrophages, the first layer of cells in the draining lymph node, act as a kind of ‘shuttle’ for HIV-1 virus-like particles. These cells help the particles spread by loading them onto two types of immune cells, follicular dendritic cells and B cells.
HIV is a virus that damages immune cells and suppresses the host’s ability to fight everyday infections and disease. During HIV-1 infection, follicular dendritic cells act as a reservoir for the virus and an obstacle to curative treatments, but it was not well known how these cells initially acquire and preserve HIV-1. Researchers from the National Institute of Allergy and Infectious Diseases (NIAID), part of the U.S. National Institutes of Health in Bethesda, Maryland, set out to investigate this further. The team consisted of John Kehrl, Chief of the B-cell Molecular Immunology Section of NIAID, and Chung Park, Staff Scientist in the same department.
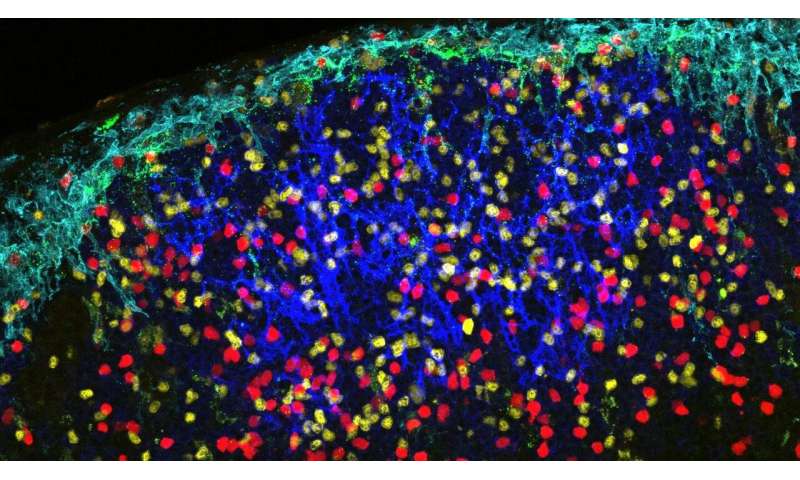
In their study, Kehrl and Park visualised the likely early events in the spread of HIV-1 virus-like particles in mice. They looked at how the virus moves from the lymph and blood via sinus-lining macrophages, and how it is then transferred to underlying networks of follicular dendritic cells.
Their work highlighted a subset of lymphoid organ sinus-lining macrophages that provide a cell-to-cell contact portal to shuttle HIV-1 particles onto follicular dendritic and B cells in the lymph node and spleen. They found that a type of protein called MFG-E8 is central to the proper function of this portal, as its absence severely limited the spread of HIV-1 onto follicular dendritic cell networks.
The team also revealed that the HIV-1 envelope, which encloses the viral particle and helps the virus enter cells, provides a means for MFG-E8 binding. MFG-E8 acts to link the HIV-1 particles to αvβ3 integrins expressed on the host’s cells. These integrins help the cell’s uptake of the viral particles, making the particles available to other cell types or, in some instances, targeting them for destruction. Kehrl and Park say that further work is now needed to see whether this process involving MFG-E8 works to benefit the host or the virus.
eLife

