New technology employing single cell genome sequencing of the parasite that causes malaria has yielded some surprising results and helps pave the way for possible new intervention strategies for this deadly infectious disease, according to Texas Biomedical Research Institute Assistant Professor Ian Cheeseman, Ph.D. Dr. Cheeseman was Principal Investigator of a three-year study published in the January 2020 edition of Cell Host & Microbe, a high-impact peer-reviewed publication.
Malaria is caused by Plasmodium parasites spread to people by the bite of infected Anopheles mosquitoes.
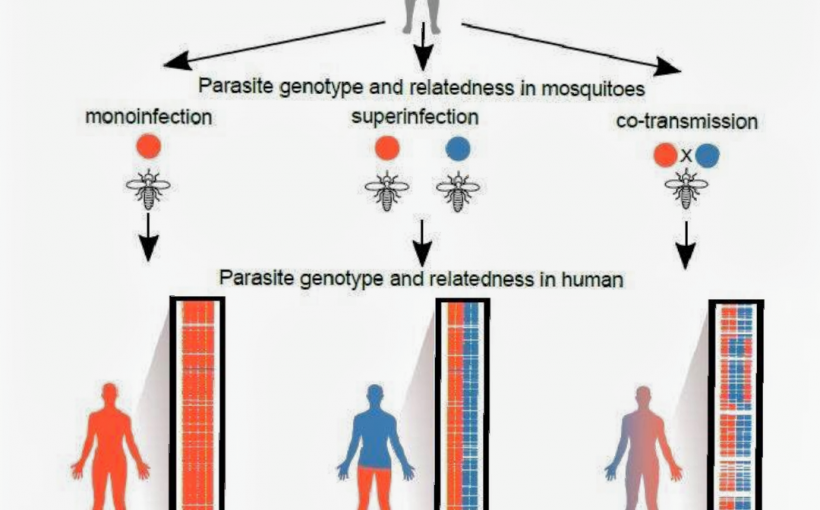
“We don’t know what is inside malaria infections,” Dr. Cheeseman said. “We don’t know how many different genetically distinct strains of parasites there are. We don’t know how related they are to each other. We don’t know how many mosquitoes they came from.”
To help answer these questions, Dr. Cheeseman and his collaborators turned to single cell genome sequencing. Using this technology, individual malaria parasite cells are isolated and their genome amplified before being analyzed by a genome sequencer. Single cell sequencing allows researchers to capture the genetic mutations present in a single cell, and has been adopted by cancer researchers to understand how tumors evolve. This is the first time the technology was used to study malaria transmission.
Dr. Cheeseman and his international team studied single malaria-infected cells from malaria patients in Malawi, a country heavily burdened by this infectious disease. Malaria patients, who donated malaria-infected blood samples used in this study reside in Chikhwawa, a region with a large mosquito population. In this region, people may be bitten by a malaria-infected mosquito every 48 hours.
The single cell sequencing approach applied in this study provides a fresh picture of how often bites from an infected mosquito lead to a malaria infection. What researchers discovered went against conventional wisdom. Nearly all the infections they studied likely came from one mosquito bite.
“We found that complex malaria infections are predominantly caused by a single mosquito bite transmitting many genetically diverse but related parasites into the blood stream of a patient,” Dr. Standwell Nkhoma, lead author on the study and a Malawian national, stated.
Knowing this enables scientists to design more effective interventions to block mosquitoes from spreading malaria and build more sophisticated models to predict the spread of antimalarial drug resistance and malaria transmission patterns. The rise of antimalarial drug resistance is a major threat to malaria control globally as resistance to the antimalarial drugs artemisinin and piperaquine continue to spread.
Malaria infects an estimated 200 million people worldwide each year and kills more than 400,000 people, most of them children, according to the World Health Organization. “Any strides we can make in understanding this disease will make an enormous impact,” Dr. Cheeseman concluded.
TEXAS BIOMEDICAL RESEARCH INSTITUTE