Johns Hopkins University
More than 35 million Americans take statin drugs daily to lower their blood cholesterol levels. Now, in experiments with human cells in the laboratory, researchers at Johns Hopkins Medicine have added to growing evidence that the ubiquitous drug may kill cancer cells and have uncovered clues to how they do it.
The findings, say the researchers, enhance previous evidence that statins could be valuable in combating some forms of cancer. In unrelated studies, other Johns Hopkins Medicine researchers have studied how statins may cut the risk for aggressive prostate cancer.
“There have been epidemiological indications that people who take statins long term have fewer and less aggressive cancers, and that statins can kill cancer cells in the laboratory, but our research was not initially designed to investigate possible biological causes of these observations,” says Peter Devreotes, Ph.D., Issac Morris and Lucille Elizabeth Hay Professor of Cell Biology.
Results of the new research appeared Feb. 12 in the Proceedings of the National Academy of Sciences.
Devreotes and his team began the new study with an unbiased screen of about 2,500 drugs approved by the U.S. Food and Drug Administration (FDA) to see which ones had the best kill rate of cells genetically engineered to have a mutation in a cancer gene called PTEN. The gene codes for an enzyme that suppresses tumor growth. Among the thousands of drugs, statins and in particular pitavastatin, emerged as a top contender in cancer-killing ability. Most of the other drugs had no effect or killed normal and engineered cells at the same rate. Equal concentrations of pitavastatin caused cell death in nearly all of the engineered cells, but very in few normal cells.
Devreotes and his team then looked at the molecular pathways that statins were likely to affect. It’s well known, for example, that statins block a liver enzyme that makes cholesterol, but the drug also blocks the creation of a small molecule called geranylgeranyl pyrophosphate, or GGPP, which is responsible for connecting cellular proteins to cellular membranes.
When the researchers added pitavastatin and GGPP to human cancer cells with PTEN mutations, the researchers found that GGPP prevented the statin’s killing effects and the cancer cells survived, suggesting that GGPP may be a key ingredient to cancer cell survival.
Next, looking under a microscope at cells engineered to lack the enzyme that makes GGPP, Devreotes and his team saw that as the cells began to die, they stopped moving. Under normal circumstances, cancer cells are a bundle of moving energy, consuming massive amounts of nutrients to maintain their unchecked growth. They maintain this breakneck pace by creating straw-like protrusions from their surface to drink up nutrients from the surrounding environment.
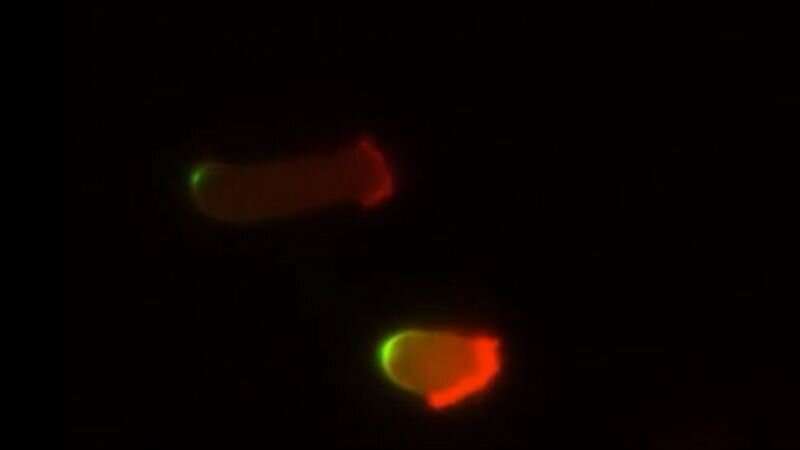
Suspecting that the non-moving cancer cells were literally “starving to death,” Devreotes says, the scientists then measured the statin-treated cells’ intake by adding a fluorescent tag to proteins in the cells’ environment.
Normal human cells glowed brightly with the fluorescent tag, suggesting that these cells ingested protein from their surroundings regardless of whether the scientists added statins to the mix of nutrients and cells. However, human cancer cells with PTEN mutations took in almost no glowing proteins after the scientists added statins. The inability of the statin-treated cancer cells to make the protrusions needed take up proteins leads to their starvation.
Devreotes says his team plans further research on the effects of statins in people with cancer and compounds that block GGPP.
Johns Hopkins University