Cardiovascular disorders are leading causes of morbidity and death worldwide. Age-associated changes in arterial properties, such as endothelial dysfunction and structural alterations, are considered to be initial events in the development of atherosclerosis. Due to the high heterogeneity of vascular cells from different sources, our understanding of human arterial aging and the development of interventions against human arterial aging and associated diseases are hindered.
Recently, scientists from the Institute of Zoology of the Chinese Academy of Sciences and Peking University have worked jointly and established the first single-cell transcriptomic atlas of primate arterial aging.
Using the single-cell transcriptome sequencing technology, they drew a comprehensive aging roadmap of cynomolgus monkey arteries at the single-cell resolution and identified FOXO3A loss as a key driver for arterial endothelial aging.
This study entitled “A single-cell transcriptomic landscape of primate arterial aging” has been published online in Nature Communications on May 5th, 2020.
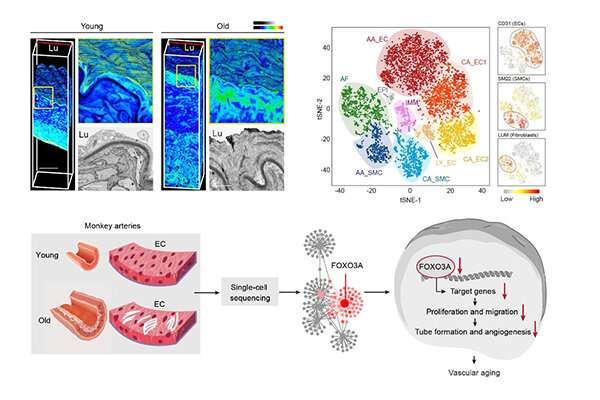
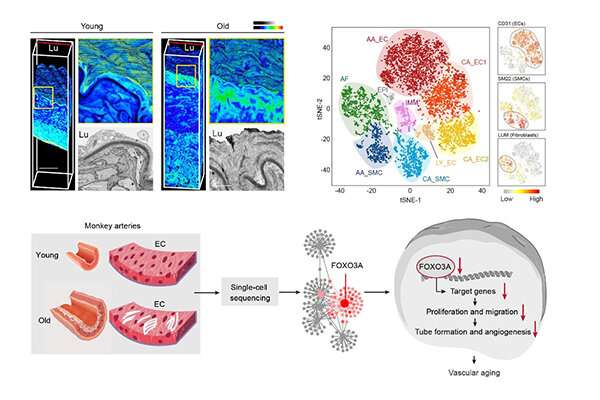
The aortic and coronary arteries are two of the most lesion-susceptible regions in the human vasculature with age. The researchers analyzed senile aortas, which exhibited characteristics of vascular aging, including increased wall thickness, fibrous cap formation, arterial calcification and elastic lamina fragmentation.
They then depicted the single-cell transcriptomic landscape of arteries from young and aged non-human primates and unraveled aorta-specific and coronary artery-specific molecular signatures for eight vascular cell types.
Gene network analyses characterized transcriptional landmarks that regulated vascular senility and positioned FOXO3A, a longevity-associated transcription factor, as a master regulator gene that was downregulated in six subtypes of monkey vascular cells during aging.
Furthermore, targeted inactivation of FOXO3A in human arterial vascular endothelial cells resulted in the disruption of cellular homeostasis and recapitulated the major phenotypic defects observed in aged monkey arteries.
Altogether, the researchers established a high-resolution single-cell transcriptomic atlas of non-human primate arterial aging, revealed the dynamic changes of cell composition and molecular characteristics during arterial aging in primates and verified FOXO3A loss as a key driver for arterial endothelial aging.
This work provides a critical resource for the understanding of primate arterial aging and contributes important clues to future treatment of age-associated vascular disorders.
Finally, it is noteworthy that the same team recently obtained the world’s first FOXO3A-enhanced human vascular cells using gene editing (Cell Stem Cell, 2019, cover article).
In contrast to the FOXO3A-deficient vascular endothelial cells mentioned above, FOXO3A-enhanced cells are significantly enhanced in proliferation, tube formation, and vascular repairability in vivo, providing an excellent graft material for the treatment of vascular degenerative diseases.
Zhang Nannan, Chinese Academy of Sciences