A study examining the effect of the immune receptor known as Toll-like Receptor 4, or TLR4, on how memory functions in both the normal and injured brain has found vastly different cellular pathways contribute to the receptor’s effects on excitability in the uninjured and injured brain.
Further, the researchers found novel mechanisms for how TLR4 regulates memory function in the normal, uninjured brain.
The study, performed on rats and published in Brain, Behavior, and Immunity, has the potential to lead to treatments aimed at limiting memory deficits after brain injury.
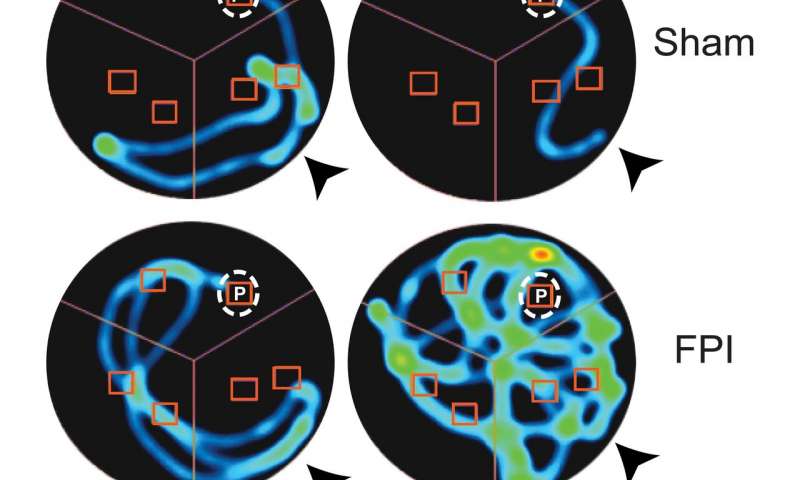
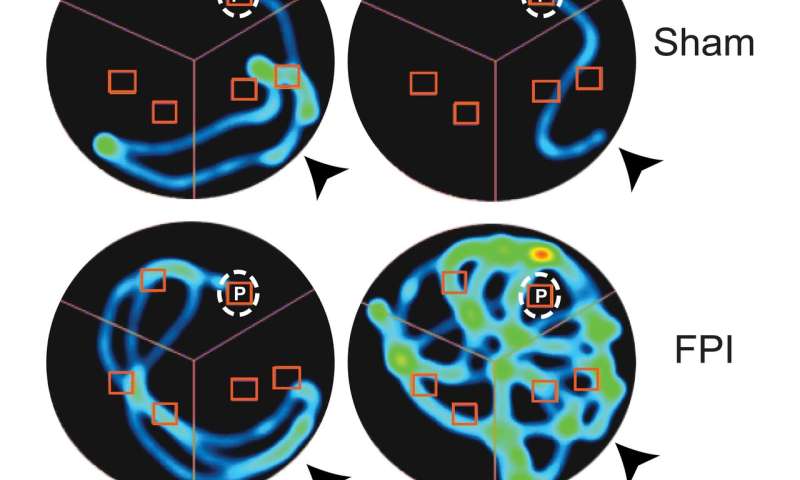
“Memory deficits are a major long term adverse consequence of brain injury and the ability of a drug treatment given a day after injury to improve memory function is of significant clinical interest,” said Viji Santhakumar, an associate professor of molecular, cell and systems biology at the University of California, Riverside, who led the study. “Resatorvid, a drug approved for clinical trials in other diseases, effectively blocked TLR4 and improved post-injury memory deficits in our study.”
The brain has neurons and non-neuronal cells called glia. In the normal brain, the activity of neurons is suppressed by TLR4; in the injured brain, TLR4 increases neuronal activity. Specifically, following brain injury, TLR4 increases excitability in the dentate gyrus, a circuit involved in memory processing in the hippocampus, the brain structure that plays a major role in learning and memory.
The UC Riverside-led study found only neurons are involved in TLR4-mediated increase in excitability in the injured brain. In contrast, glial cells are necessary for TLR4-mediated reduction of excitability in the normal brain.
“We found suppressing TLR4 signaling a day after concussive brain injury, common to sports and traffic accidents, reduces excitability and improves working memory performance a week to a month later,” she said. “Our data show the processes underlying the damaging effects of brain injury can be selectively manipulated for therapy and can preserve memory function after the injury.”
How exactly TLR4 affects memory function in the normal and injured brain is not clear.
“While the actual mechanisms are not known, the speculation is that TLR4 regulates memory function by suppressing neuronal activity in the normal brain, thereby improving signal-to-noise ratio,” Santhakumar said. “After injury, the effect of TLR4 flips to enhancing neuronal activity and promoting excitability, which increases noisy and non-specific neuronal firing and degrades signal-to-noise. It is possible that after injury TLR4 impairs cellular processes involved in memory formation, which my lab is currently investigating. The bottom line is that inhibiting TLR4 signaling in the injured brain leads to improvements in long-term working memory deficits for weeks to a month.”
Santhakumar was intrigued by a common immune signaling molecule, TNFα, which contributes to both TLR4-dependent reduction in excitability in normal, uninjured brains, and TLR4-mediated increase in excitability after concussive brain injury. In the normal brain, glial cells are involved; in the injured brain, neurons are involved.
“Our study has identified a novel role for neuronal TNFα in regulating excitability,” she said. “We were surprised that glial versus neuronal signaling mediated by the same signaling molecule, TNFα, has different effect on neuronal excitability.”
Next, Santhakumar and her team plan to determine how TLR4 signaling through glial mediators suppresses network excitability and facilitates memory performance in the normal brain. The researchers also plan to explore how neuronal TLR4 signaling after injury can be selectively manipulated.
“We are interested in determining if blocking TLR4 signaling after injury prevents network reorganization and abnormal changes in rhythmic or oscillatory brain activity that is crucial to memory processing,” she said.
The title of the research paper is “Distinct cellular mediators drive the Janus faces of toll-like receptor 4 regulation of network excitability which impacts working memory performance after brain injury.”
Iqbal Pittalwala, University of California – Riverside