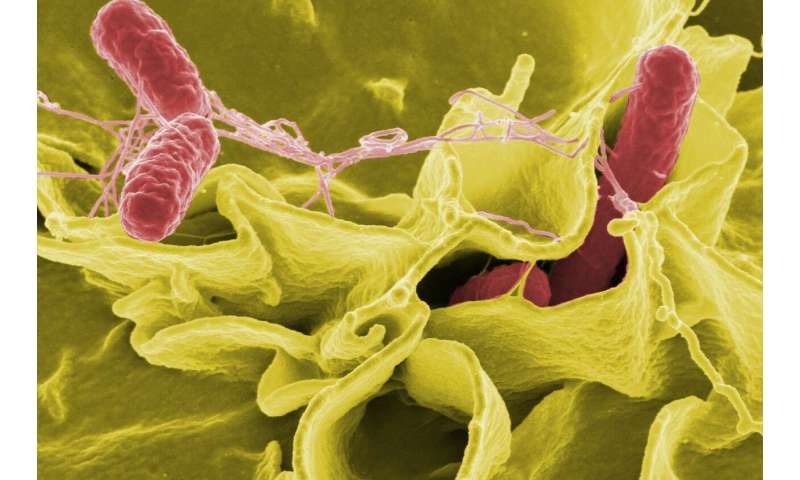
Melbourne researchers have revealed the multiple, intertwined cell death systems that prevent the spread of the ‘intracellular’ bacterium Salmonella, an important cause of typhoid fever which kills more than 100,000 people annually.
The team revealed that the spread of Salmonella is curtailed by the death of infected cells, but surprisingly cells can die in several distinct ways. Although Salmonella continuously seeks to outsmart infected cells by blocking their suicide, cells have evolved impressive ‘back-up’ strategies to ensure that the infected cell can still die and thus protect the body from Salmonella infection and consequent typhoid fever.
The research, published in the journal Immunity, was led by a collaborative team including Walter and Eliza Hall Institute researchers Dr. Marcel Doerflinger, Ms Yexuan Deng, Dr. Ranja Salvamoser, Associate Professor Marco Herold and Professor Andreas Strasser, and University of Melbourne Professor Sammy Bedoui and Dr. Paul Whitney, researchers from the Peter Doherty Institute for Infection and Immunity (Doherty Institute).
Fighting the enemy within
“Many disease-causing bacteria invade cells, surviving and reproducing within the cells and hiding from the body’s immune system. Salmonella, a cause of serious food-borne infections, is one such ‘intracellular’ bacterium. Cells have developed a range of defenses against intracellular bacteria,” Professor Bedoui said.
“The rapid death of infected cells is an important protective strategy against intracellular bacteria. This stops the reproduction and spread of the bacteria, and can trigger protective immune defenses at the site of the infection, which further control the infection,” he said.
“Many proteins have been thought to be important for driving the death of bacteria-infected cells, which signal within cells and also degrade key components of the cell to bring about its death. However, there has been uncertainty about precisely how bacteria-infected cells die, the key molecules involved, and what this means for controlling an infection,” Professor Bedoui said.
Backup cell death pathways
“The team used laboratory models lacking different combinations of cell death proteins to understand their contribution to the control of Salmonella infections,” Associate Professor Herold said.
“We investigated the roles of proteins involved in three key types of cell death: apoptosis, pyroptosis and necroptosis,” he said. “While these processes all result in cell death, each occurs differently at the molecular level, and has different consequences for triggering immunity and inflammation.”
“When only one of the three forms of cell death were disabled, there was only a minor impact on how effectively Salmonella infections were controlled—this showed that cells were not reliant on one specific system,” Dr. Doerflinger said.
“When we disabled two or all three forms of cell death, we saw that Salmonella infections were not controlled and the bacteria rapidly spread. This suggested that cells have developed several ‘backups’ to ensure cell death happens if there is a fault in one cell death pathway. While we only studied Salmonella, we speculate that our findings might be relevant to other intracellular pathogens such as the bacterium that causes tuberculosis,” he said.
“The team also revealed unexpected roles for cell death proteins called caspases,” Professor Strasser said. “Until now, certain caspases including two known as ‘caspase 1’ and ‘caspase 8’ had very well-defined roles as early triggers of two distinct types of cell death. Our results showed that contrary to current perceptions these caspases can act within the ‘other pathway’ and even at later, critical stages of cell death when the cell is being dismantled.
“This is an example of another fail-safe process in the overall cell death machinery that ensures protection against pathogens like Salmonella,” he said.
The flexibility in how cells can die can be explained by the ongoing battle between animals and disease-causing bacteria.
“Throughout evolution, both sides have developed new tactics in an ‘arms race’ for supremacy. Living and multiplying inside cells—rather than outside—helped the bacteria avoid immune detection, but animals responded by developing ways for infected cells to undergo altruistic suicide—which we have revealed is a highly coordinated but flexible system that has several fail-safe mechanisms,” Professor Strasser said.
Walter and Eliza Hall Institute

