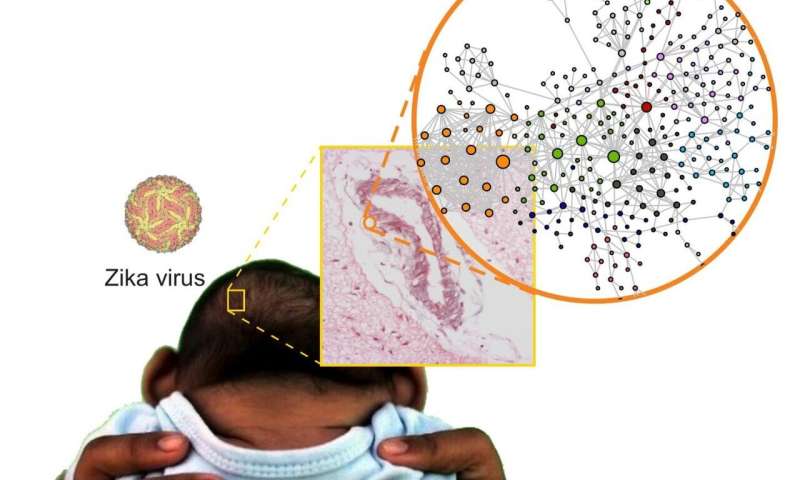
Zika virus infection during pregnancy can cause severe abnormalities in the fetus, including malformations such as microcephaly. In a small proportion of cases, the disease may lead to miscarriage and perinatal death. A network of more than 30 Brazilian researchers set out to find the causes of these problems with the support of FAPESP and obtained important results after half a decade of hard work. A paper describing their findings has been published in the journal Science Signaling.
“We show for the first time what happens in the fetal brain affected by congenital Zika syndrome [CZS],” Helder Nakaya, who is the last author of the paper, told Agência FAPESP. Nakaya is a bioinformatics specialist, a professor at the University of São Paulo’s School of Pharmaceutical Sciences (FCF-USP), and a senior scientist at the Center for Research on Inflammatory Diseases (CRID), which is one of the Research, Innovation and Dissemination Centers (RIDCs) funded by FAPESP.
The researchers compared brain tissue from babies who died from CZS with tissue samples from babies that died from other causes. “The parents who gave us permission to collect these samples at a time of such grief were exceptionally altruistic. They were motivated by the knowledge that this donation helped science and that science could help others in future,” Nakaya said.
The comparison revealed several anomalies in the brains of the babies with CZS. “Analysis of the brain genome [all DNA], transcriptome [RNAs transcribed from genes] and proteome [proteins produced using messenger RNAs] showed a number of significant molecular alterations in genes related to neuron development, the possible dysregulation of neurotransmitters such as glutamate and even alterations in different types of collagen,” Nakaya said.
The researchers integrated transcriptomics and proteomics data to identify microRNAs (miRNAs) that do not encode proteins but regulate gene expression and may be linked to CZS. One of these, mir-17-5p, was found in previous research to be associated with viral infection in cultured astrocytes, the most abundant type of central nervous system cell.
“Other important findings include genetic variants of key proteins involved in the development of the immune system and nervous system,” Nakaya said. “These findings may explain the increased susceptibility to CZS in babies that have these genetic variants. Finally, when we integrated all three types of data [genomics, transcriptomics and proteomics], we found alterations in signaling pathways related to the organization of the extracellular matrix, which may partly explain the features of CZS.” The extracellular matrix is an array of macromolecules secreted by cells that acts as a structural scaffold that regulates cell differentiation and tissue growth and contributes to organ maintenance, among other functions.
The bioinformatics part of the study was intense because of the enormous volume of data generated, according to Nakaya. “Human DNA contains 3.2 billion base pairs, which can generate 150,000 transcripts [both protein-encoding and noncoding RNAs] and encode more than 25,000 proteins,” he said. “Integration of so much biological information was possible only by a multidisciplinary team that included scientists from several excellent research institutions.”
Science takes time, and this is not always understood, Nakaya added. “The Zika outbreak began in 2015, and only now do we have these results. Scientific research cannot be performed overnight. I know everyone wants answers quickly, but the fact is that if you speed up the process artificially, you risk getting bad science,” he said.
The raw data are publicly available so that the scientific community can perform their own analyses and investigate in depth the role of every molecule described in the paper.
José Tadeu Arantes , FAPESP

