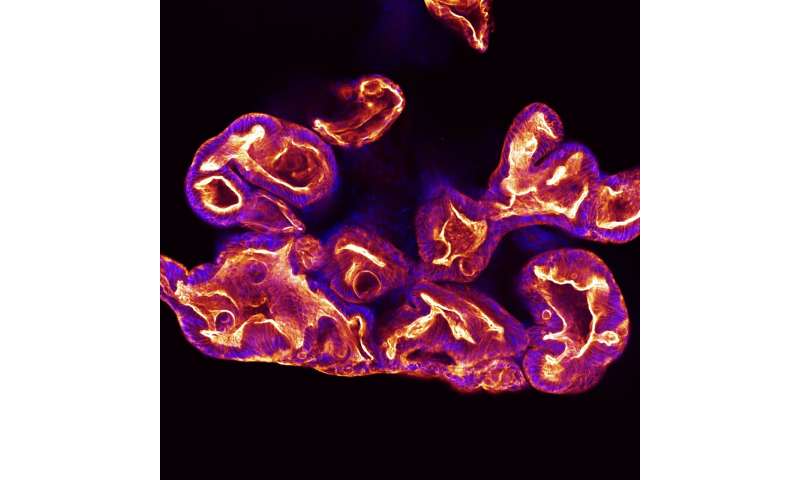
Organoids are stem cell-based tissue surrogates that can mimic the structure and function of organs, and they have become a key component of numerous types of medical research in recent years. But researchers from The University of Texas at Austin have uncovered problems with the conventional method for growing organoids for common experiments that may cause misleading results.
The researchers discovered that the size of organoids differ depending on where they are located within the hydrogel material called extracellular matrix (ECM) that is commonly used in biomedical research. The team found that organoids on the edges of a dome-shaped ECM respond differently to chemical or biological stimuli compared to those in the center of the dome.
This observation means one organoid in the core might react positively to a new treatment or drug, while another one on the edge could have a negative reaction, potentially muddying the results of an experiment. Ideally, organoids would be consistent in size and reaction in preclinical experiments.
“There are hundreds of organoids in the hydrogel dome, and they’re showing different sizes, different functions, and that can be problematic” said Woojung Shin, a postdoctoral fellow and recent Ph.D. graduate from the Cockrell School of Engineering’s Department of Biomedical Engineering, who discovered the problem. “You may get very different results from what would actually happen in the human body as a result.”
The findings were published recently in Cell Press’ iScience. The team includes researchers from the Biomimetic Microengineering Laboratory in the Cockrell School and the Livestrong Cancer Institutes of UT’s Dell Medical School.
The research began when Shin noticed that something felt off while examining organoids. They were slightly different sizes based on their location in the sample.
They repeated the experiment and identified the same issues time after time with different organoid lines. The team found morphogens in the culture medium—signaling molecules that are essential for organoid growth—that can spread and create a “gradient” within the hydrogel domes. One of the representative morphogens, Wnt3a, was extremely unstable. A computational simulation confirmed that the size difference in organoids is likely explained by the morphogen gradient and its instability.
The paper mainly focuses on the problem the researchers uncovered, but it also offers a roadmap for finding solutions. The key, the researchers say, is to stabilize the Wnt3a protein across the sample, reducing the size of the gradient created and, subsequently, the location-based differences in the organoids.

Shin is a member of biomedical engineering assistant professor Hyun Jung Kim’s research group. She focuses on disease modeling and bioinspired organ mimicry.
Organoids are an important part of the ongoing research conducted by Kim and his group. The team uses nature’s engineering principles, or biomimetic engineering, to solve the fundamental questions about human health and disease, most notably through its organ-on-a-chip technology.
Continuing to refine organoid research principles is key to the success of Kim’s group as well as a host of different types of medical research. The paper mentions disease modeling, tissue engineering, patient-specific validation of new drug candidates and research into the relationship between demographics and disease as areas that have benefitted from organoid research.
“We really want to have reproducible and reliable experimental results,” Kim said. “What we’ve found here is that we all need to be more cautious about how we interpret data, and then maybe we can decrease the risk of misinterpretation.”
University of Texas at Austin

