A research collaboration between Kumamoto University and Nagasaki University in Japan has found that components leaking from broken muscle fibers activate “satellite” muscle stem cells. While attempting to identify the proteins that activate satellite cells, they found that metabolic enzymes, such as GAPDH, rapidly activated dormant satellite cells and accelerated muscle injury regeneration. This is a highly rational and efficient regeneration mechanism in which the damaged muscle itself activates the satellite cells that begin the regeneration process.
Skeletal muscle is made up of bundles of contracting muscle fibers and each muscle fiber is surrounded by satellite cells—muscle stem cells that can produce new muscle fibers. Thanks to the work of these satellite cells, muscle fibers can be regenerated even after being bruised or torn during intense exercise. Satellite cells also play essential roles in muscle growth during developmental stages and muscle hypertrophy during strength training. However, in refractory muscle diseases like muscular dystrophy and age-related muscular fragility (sarcopenia), the number and function of satellite cells decreases. It is therefore important to understand the regulatory mechanism of satellite cells in muscle regeneration therapy.
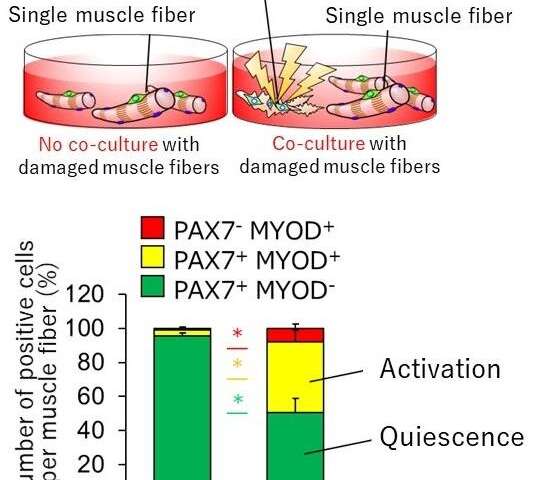
In mature skeletal muscle, satellite cells are usually present in a dormant state. Upon stimulation after muscle injury, satellite cells are rapidly activated and proliferate repeatedly. During the subsequent myogenesis, they differentiate and regenerate muscle fibers by fusing with existing muscle fibers or with together. Of these three steps (satellite cell activation, proliferation, and muscle differentiation), little is known about how the first step, activation, is induced.
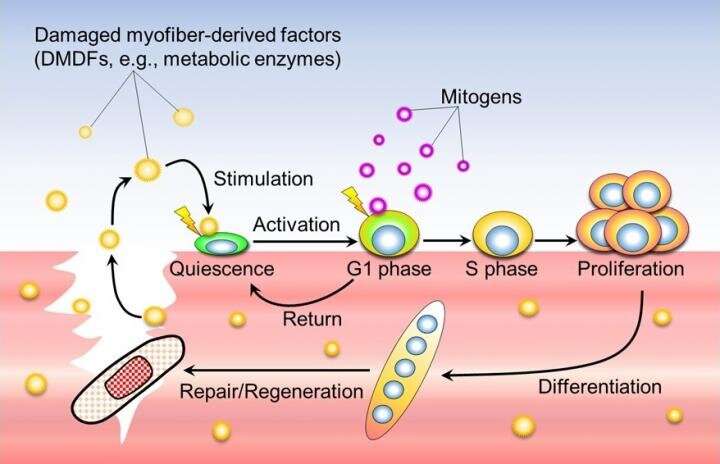 Factors (DMDFs) leaking from the injured muscle fiber quickly activate dormant satellite cells, which then enter the preparatory phase for proliferation (G1 phase). Subsequently, by stimulation of growth factors produced by immune cells and stromal cells infiltrating the injured area, satellite cells enter the S phase and proliferate. DMDFs are thought to be the mechanism that activates satellite cells enabling rapid muscle regeneration after injury. Credit: Associate Professor Yusuke Ono
Factors (DMDFs) leaking from the injured muscle fiber quickly activate dormant satellite cells, which then enter the preparatory phase for proliferation (G1 phase). Subsequently, by stimulation of growth factors produced by immune cells and stromal cells infiltrating the injured area, satellite cells enter the S phase and proliferate. DMDFs are thought to be the mechanism that activates satellite cells enabling rapid muscle regeneration after injury. Credit: Associate Professor Yusuke Ono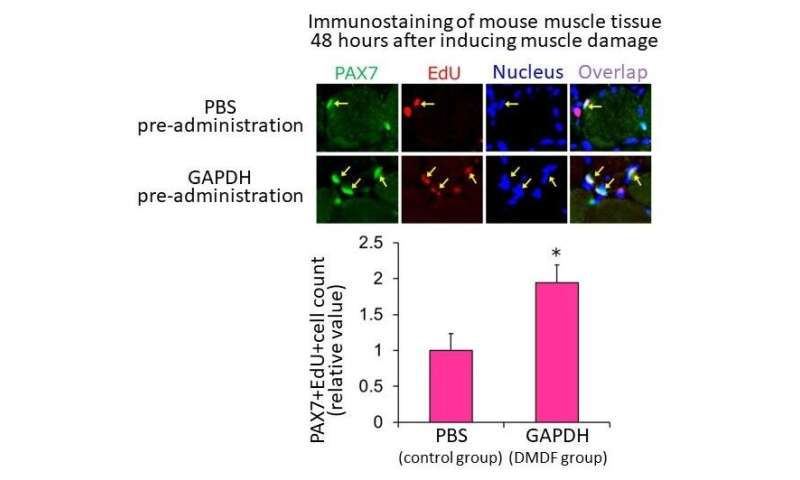 In a mouse muscle damage model, GAPDH recombinant protein was injected into the tibialis anterior muscle 24 hours before muscle damage was induced by drug injection (DMDF group). Phosphate buffered saline (PBS) was administered to the control group. The number of EdU-positive cells increased in the DMDF group compared to the control group, as assessed by immunohistochemical staining of muscle tissue 48 hours after muscle injury. Credit: Associate Professor Yusuke Ono
In a mouse muscle damage model, GAPDH recombinant protein was injected into the tibialis anterior muscle 24 hours before muscle damage was induced by drug injection (DMDF group). Phosphate buffered saline (PBS) was administered to the control group. The number of EdU-positive cells increased in the DMDF group compared to the control group, as assessed by immunohistochemical staining of muscle tissue 48 hours after muscle injury. Credit: Associate Professor Yusuke Ono Factors (DMDFs) leaking from the injured muscle fiber quickly activate dormant satellite cells, which then enter the preparatory phase for proliferation (G1 phase). Subsequently, by stimulation of growth factors produced by immune cells and stromal cells infiltrating the injured area, satellite cells enter the S phase and proliferate. DMDFs are thought to be the mechanism that activates satellite cells enabling rapid muscle regeneration after injury. Credit: Associate Professor Yusuke Ono
Factors (DMDFs) leaking from the injured muscle fiber quickly activate dormant satellite cells, which then enter the preparatory phase for proliferation (G1 phase). Subsequently, by stimulation of growth factors produced by immune cells and stromal cells infiltrating the injured area, satellite cells enter the S phase and proliferate. DMDFs are thought to be the mechanism that activates satellite cells enabling rapid muscle regeneration after injury. Credit: Associate Professor Yusuke Ono In a mouse muscle damage model, GAPDH recombinant protein was injected into the tibialis anterior muscle 24 hours before muscle damage was induced by drug injection (DMDF group). Phosphate buffered saline (PBS) was administered to the control group. The number of EdU-positive cells increased in the DMDF group compared to the control group, as assessed by immunohistochemical staining of muscle tissue 48 hours after muscle injury. Credit: Associate Professor Yusuke Ono
In a mouse muscle damage model, GAPDH recombinant protein was injected into the tibialis anterior muscle 24 hours before muscle damage was induced by drug injection (DMDF group). Phosphate buffered saline (PBS) was administered to the control group. The number of EdU-positive cells increased in the DMDF group compared to the control group, as assessed by immunohistochemical staining of muscle tissue 48 hours after muscle injury. Credit: Associate Professor Yusuke Ono
Since satellite cells are activated when muscle fibers are damaged, researchers hypothesized that muscle damage itself could trigger activation. However, this is difficult to prove in animal models of muscle injury so they constructed a cell culture model in which single muscle fibers, isolated from mouse muscle tissue, were physically damaged and destroyed. Using this injury model, they found that components leaking from the injured muscle fibers activated satellite cells, and the activated cells entered the G1 preparatory phase of cell division. Further, the activated cells returned to a dormant state when the damaged components were removed, thereby suggesting that the damaged components act as the activation switch.
The research team named the leaking components damaged myofiber-derived factors (DMDFs), after the broken muscle fibers, and identified them using mass spectrometry. Most of the identified proteins were metabolic enzymes, including glycolytic enzymes such as GAPDH, and muscle deviation enzymes that are used as biomarkers for muscle disorders and diseases. GAPDH is known as a “moonlighting protein” that has other roles in addition to its original function in glycolysis, such as cell death control and immune response mediation. The researchers therefore analyzed the effects of DMDFs, including GAPDH, on satellite cell activation and confirmed that exposure resulted in their entry into the G1 phase. Furthermore, the researchers injected GAPDH into mouse skeletal muscle and observed accelerated satellite cell proliferation after subsequent drug-induced muscle damage. These results suggest that DMDFs have the ability to activate dormant satellite cells and induce rapid muscle regeneration after injury. The mechanism by which broken muscle activates satellite cells is a highly effective and efficient tissue regeneration mechanism.
“In this study, we proposed a new muscle injury-regeneration model. However, the detailed molecular mechanism of how DMDFs activate satellite cells remains an unclear issue for future research. In addition to satellite cell activation, DMDF moonlighting functions are expected to be diverse,” said Associate Professor Yusuke Ono, leader of the study. “Recent studies have shown that skeletal muscle secretes various factors that affect other organs and tissues, such as the brain and fat, into the bloodstream, so it may be possible that DMDFs are involved in the linkage between injured muscle and other organs via blood circulation. We believe that further elucidation of the functions of DMDFs could clarify the pathologies of some muscle diseases and help in the development of new drugs.”
Kumamoto University

