In a first for science, researchers at the Stanford School of Medicine have assembled a working model of the human nerve-cell circuit responsible for voluntary movement.
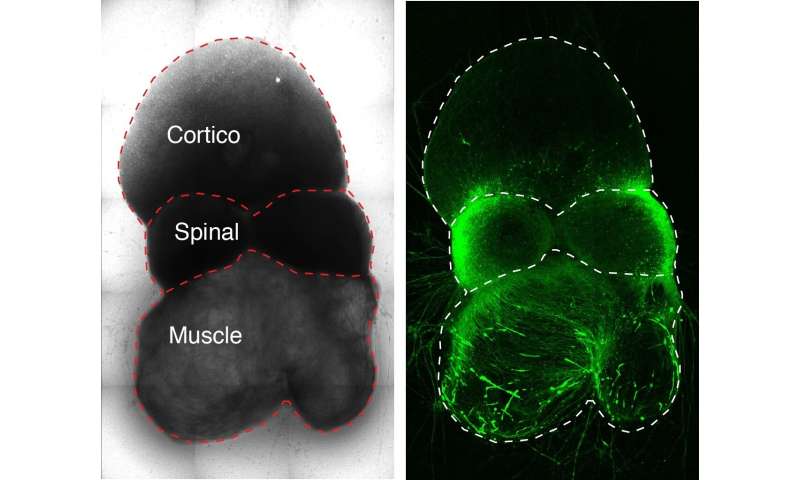
The researchers generated the circuit’s three component pieces—tissue representing the cerebral cortex, spinal cord and skeletal muscle—from human stem cells and let them sew themselves together in a dish.
The advance promises to accelerate the study of a wide variety of neurological conditions. It also demonstrates that the brain can fuse with a body part in laboratory glassware to form a nerve circuit that works the way it does in real life.
A disruption in the nerve circuits that relay commands from the brain to the skeletal muscles can lead to immobility and death. Scientists have studied lab animals to try to understand neuromuscular impairment in humans, but their efforts have been hobbled by interspecies differences in nerve circuitry.
“ALS has been cured in rodents dozens of times,” said Sergiu Pasca, MD, associate professor of psychiatry and behavioral sciences, referring to amyotrophic lateral sclerosis, or Lou Gehrig’s disease. “None of the cures have effectively translated to people. But now we can use patients’ own cells to generate personalized working models that will help us study these disorders in a dish.”
Assembloids
The Stanford team created their roughly third-of-an-inch-long models, which Pasca calls assembloids, from human induced pluripotent stem cells, or iPS cells. The work is described in a study published online Dec. 16 in Cell. Pasca, the Bonnie Uytengsu and Family Director of the Stanford Brain Organogenesis Program, is the study’s senior author. The lead author is research scientist Jimena Anderson, Ph.D.
In past work, Pasca’s group has generated miniature spheroids that closely mimic the architecture and physiology of the cerebral cortex. In the new study, the scientists tweaked their recipe to generate two additional types of spheroids approximating spinal-cord tissue and skeletal muscle.
These three spheroid types represent the three critical components of the circuit controlling voluntary movement. Placed next to one another in a dish, with the spinal spheroid in the center, the spheroids gradually fused together.
Pasca and his colleagues demonstrated that the circuit assembled accurately: Neurons from the cortical spheroid hooked up with spinal-spheroid motor neurons, which innervated muscle tissue.
“We made the parts,” Pasca said, “but they knew how to put themselves together.”
Triggering a muscle twitch
But were these, indeed, working connections? Would stimulating cortical nerve cells cause the muscle tissue to contract?
Using laboratory techniques, the scientists showed that stimulating the cortical spheroids triggered a twitch in the skeletal-muscle spheroids.
“Skeletal muscle doesn’t usually contract on its own,” Pasca said. “Seeing that first twitch in a lab dish immediately after cortical stimulation is something that’s not soon forgotten.”
The assembloids remain fully functional for at least 10 weeks after fusing together, Pasca said. “Remarkably, the longer they remain intact, the better the contraction is,” he said.
Pasca’s group is collaborating with a team led by Jan Carette, Ph.D., associate professor of microbiology and immunology, to use the assembloids to unravel the pathology of poliovirus and other crippling poliolike viruses, whose receptors are present only in primates.
There’s no obvious limit on how many combinations are possible with spheroids. In a study published Dec. 3 in Nature Biotechnology, Pasca’s team fused a cortical spheroid to another spheroid representing the striatum, a brain structure that’s the hub of pleasurable sensations and motivated behaviors. This assembloid may prove useful for studying the causes of schizophrenia, depression and addiction, Pasca said.
Bruce Goldman, Stanford University Medical Center

