Hepatocellular carcinoma (HCC), frequently seen in patients with liver cirrhosis caused by alcohol abuse or chronic viral hepatitis, is the most common form of liver cancer worldwide. As such, it is the third-most common cause of cancer-related death and has a notoriously poor prognosis. At present, surgery is the most effective treatment for HCC, but is only successful in the 10%–20% of cases where cancer cells have not spread beyond the liver.
Given the lack of treatment options for HCC, a group of researchers led by Osaka University decided to focus on specific cells and processes that occur in the area around liver tumors in the hope of finding a novel target for drug development.
The results of their study were published in a recent issue of Gastroenterology.
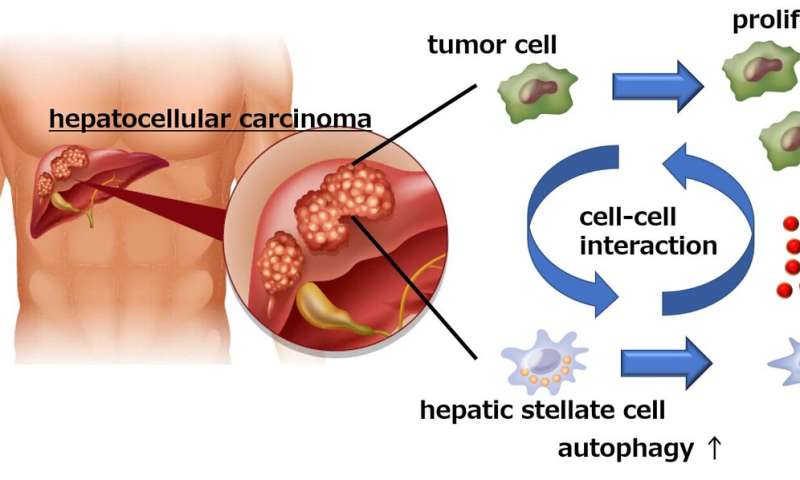
“Hepatic stellate cells (HSCs) are normal liver cells that play a role in the formation of scar tissue in response to liver damage,” explains co-author of the study Hayato Hikita. “High levels of activated HSCs have been reported in the tumor microenvironment and are associated with a poor prognosis in HCC patients. However, no one had examined the interaction between HSCs and cancer cells in the liver.”
When the researchers cultured liver cancer cells together with HSCs, they observed a significant increase in the number of cancer cells, suggesting that the HSCs somehow promoted cancer cell growth. Interestingly though, inhibition of autophagy (a cellular process primarily designed to remove damaged or unwanted cellular components) in the HSCs prevented the proliferation of cancer cells.
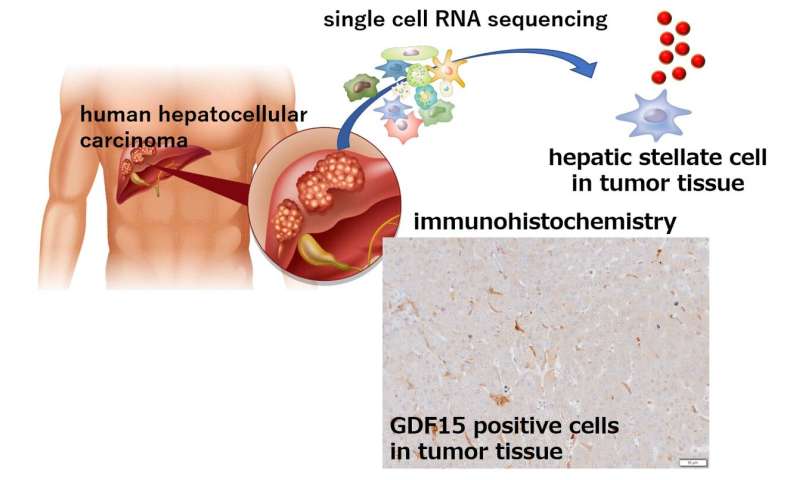 Fig3. GDF15 positive hepatic stellate cells exist in human hepatocellular carcinoma. Credit: Osaka University
Fig3. GDF15 positive hepatic stellate cells exist in human hepatocellular carcinoma. Credit: Osaka University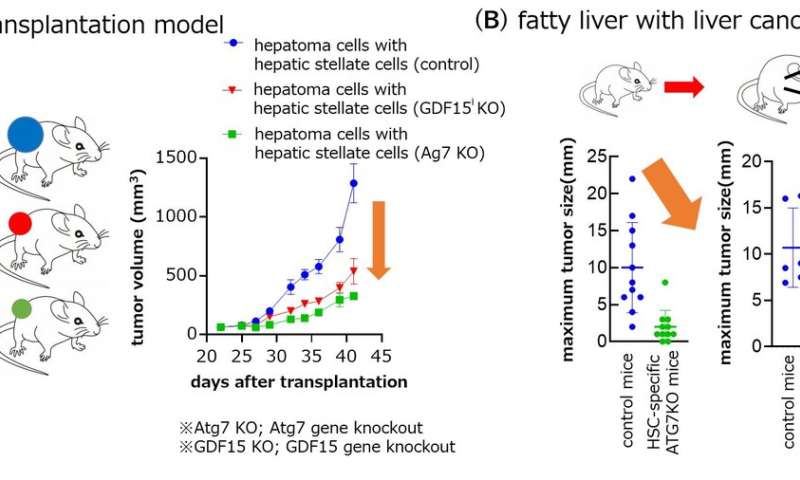 Fig2. The liver tumor growth was attenuated by knockout Atg7 or GDF15 in hepatic stellate cells. Credit: Osaka University
Fig2. The liver tumor growth was attenuated by knockout Atg7 or GDF15 in hepatic stellate cells. Credit: Osaka University Fig3. GDF15 positive hepatic stellate cells exist in human hepatocellular carcinoma. Credit: Osaka University
Fig3. GDF15 positive hepatic stellate cells exist in human hepatocellular carcinoma. Credit: Osaka University Fig2. The liver tumor growth was attenuated by knockout Atg7 or GDF15 in hepatic stellate cells. Credit: Osaka University
Fig2. The liver tumor growth was attenuated by knockout Atg7 or GDF15 in hepatic stellate cells. Credit: Osaka University
Using a mouse model of liver cancer and an analysis of gene expression, the researchers made the startling discovery that the cancer cells actually induced autophagy in the HSCs, which in turn caused the HSCs to secrete a protein called GDF15, which promoted tumor growth.
“When we examined liver samples from HCC patients with and without tumors, we found that the tumor tissue samples had much higher levels of GDF15,” says senior author Tetsuo Takehara. “Most importantly though, when we then examined the association between GDF15 expression and clinical outcome, we found that patients with higher levels of GDF15 had a poorer prognosis than those with only low levels of GDF15 expression, which really highlighted the role of GDF15 in HCC progression.”
Building on the findings of this study, novel therapies targeting GDF15 expression by HSCs are an exciting new prospect for the treatment of HCC.
Osaka University

