The human heart works under high demand, constantly pumping oxygen-rich blood through the body. When faced with disease, however, fulfilling this demand can become increasingly difficult and harmful. In the case of chronic high blood pressure—a leading cardiovascular disease in the United States—the heart continuously overexerts, resulting in maladaptive growth and, ultimately, severe dysfunction of the heart muscle itself.
Maladaptive growth of the heart, known as cardiac hypertrophy, is brought about in part by activation of G protein-coupled kinase 5 (GRK5), a signaling molecule found within heart cells that previously has been linked to worsened cardiac function in heart failure. Researchers at the Lewis Katz School of Medicine at Temple University (LKSOM) now show for the first time in animals that keeping GRK5 out of the heart cell nucleus, the compartment that houses the cell’s genes, can block this abnormal growth process.
The new findings, published online March 30 in the journal Science Signaling, open exciting avenues for the development of GRK5-based therapies to prevent heart failure, a disease in which the heart is no longer able to pump blood through the body. Heart failure typically develops after years of the heart compensating for the effects of high blood pressure.
“GRK5 acts as a pathological gene regulator in the heart, with its activity specifically in the heart cell nucleus being a major factor driving cardiac hypertrophy in heart failure,” explained Walter J. Koch, Ph.D., W.W. Smith Endowed Chair in Cardiovascular Medicine, Professor and Chair of the Department of Pharmacology, Director of the Center for Translational Medicine at LKSOM, and senior investigator on the new study.
GRK5 normally hangs out in the cell membrane. But in the heart, in response to hypertrophic stress, it translocates to the nucleus, binds to certain factors that regulate genes, and thereby triggers the production of proteins involved in tissue growth.
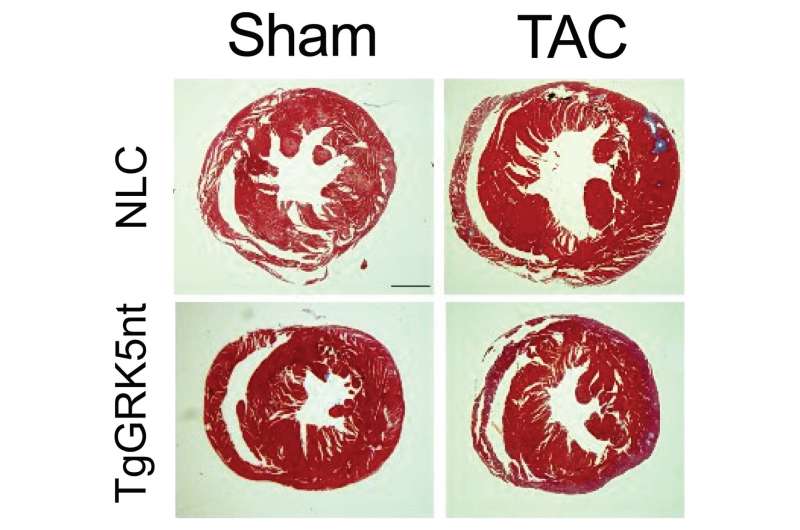
To assess the possibility of preventing this GRK5-driven abnormal growth of heart tissue, Dr. Koch’s team developed a novel peptide molecule that encodes a portion of the GRK5 molecule involved in nuclear translocation. The peptide, named GRK5nt, inhibited GRK5 entry into the nucleus, blocking its growth-signaling capabilities.
Dr. Ryan C. Coleman, a former graduate student in the Koch laboratory and first author on the report, carried out initial experiments with peptide in heart cells cultured in vitro. These experiments showed that the peptide’s expression attenuates the activation of genes involved in maladaptive stress responses. In vitro studies further confirmed that GRK5nt works by binding to and occupying a portion of the GRK5 molecule known as the calmodulin (CaM) domain, which GRK5 otherwise depends on to gain entry into the nucleus.
The researchers next tested the effects of nuclear targeting in a mouse model in which animals were engineered to express GRK5nt in the heart. Some animals further underwent surgical aortic constriction, a procedure that mimics pressure overload in the human heart. Relative to animals with normal GRK5, those expressing GRK5nt exhibited reduced maladaptive effects, including reduced hypertrophy, in response to pressure overload.
“The reduction in pathological signaling provides new insights into the possibility of designing therapies to specifically target the pathological nuclear signaling of GRK5, while preserving normal GRK5 function in other tissues,” Dr. Coleman said.
“The findings are very exciting, since they show that by keeping GRK5 out of the nucleus, we can stop abnormal growth and progression of heart failure,” Dr. Koch added.
Now with proof-of-principle that nuclear targeting can eliminate the pathological effects of GRK5, Dr. Koch and colleagues plan next to test their strategy in an animal model of heart failure. In preparation for therapeutic testing, they also plan to explore mechanisms for targeted delivery of the peptide to the heart.
Temple University

