It turns out that not all build-ups of tau protein are bad, and a team of researchers from the Perelman School of Medicine at the University of Pennsylvania developed a method to show that. Using mammalian cell models, the researchers combined extremely high-resolution microscopy with machine learning to show that tau actually forms small aggregates when a part of the body’s normal physiology. Through this, they could distinguish between the aggregates occurring under healthy conditions from the ones associated with neurological diseases, potentially opening the door to screening for treatments that might break apart harmful aggregates. This research was published in the Proceedings of the National Academy of Sciences.
“There aren’t many tools that can visualize small, pathological protein aggregates within cells,” said the study’s senior author, Melike Lakadamyali, Ph.D., an associate professor of Physiology. “But through machine learning informed by super-resolution microscopy, we believe we’ve been able to show that tau forms both normal physiological aggregates and distinct pathological aggregates. In doing so, we created a useful method that could be the basis for new research into the appropriate treatments for tau-related pathologies.”
Tau is a protein that attaches to the microtubule structure of axons—which act much like highways in nerve cells. Previously, tau aggregates had been thought to only form once tau falls off the microtubules. These have been associated with some neurological diseases, including Alzheimer’s and other types of dementia. However, it turns out that small tau aggregates can also form outside disease conditions.
“Intrinsically, there is value in being able to tell which tau aggregates are a part of a healthy person’s nervous system, and which have formed harmful aggregates,” said the study’s lead author, Melina Theoni Gyparaki, a doctoral student in Lakadamyali’s lab. “Unfortunately, there has not been a process sensitive enough to make that distinction yet inside cells. So we set out to create one using mammalian cell models.”
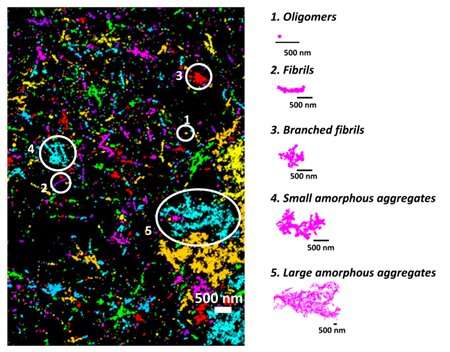
First, the researchers used extremely high resolution microscopes capable of looking at single molecules to differentiate physiological and pathological oligomers (molecular formations). Monomers, dimers and trimers, which are oligomers made up of one, two, and three tau molecules, respectively, were most likely to end up associated with healthy physiological conditions because they were associated with microtubules and regular function.
When the team looked at tau structures associated with a mammalian cell model approximating frontotemporal dementia with Parkinsonism linked to chromosome 17 (FTDP-17) – a disease associated with tau aggregation—the structures were larger and more complex. These appeared to be the pathological tau aggregates that broke off.
With the differences in configuration established, the researchers created a machine learning algorithm to classify the pathological tau aggregates by shape alone. Additionally, they used antibodies that can detect and differentiate when the tau aggregates become “hyper-phosphorylated”—when they pick up a lot of phosphate groups and tend to harmfully break off. Combining these methods showed that tau containing phosphate groups on certain amino-acids was more likely to form linear fibrils, a thin structure, as opposed to other shapes of tau aggregates.
“The method we developed to identify tau aggregates is not yet a diagnostic tool, but we think it would be a useful research tool for anyone interested in studying the mechanisms that lead to pathological protein oligomerization in neurodegenerative disease,” Lakadamyali said.
Tau aggregates aren’t the only ones that this method could be used to classify, either. There’s an opportunity to use it on other potentially pathological protein build-ups, such as alpha-synuclein, which is associated with Parkinson’s disease, or huntingtin, related to Huntington’s disease. It could also be used to screen for potential treatments for these conditions that don’t harm the body’s regular protein complexes.
The team is now studying potential mechanisms for clearing tau aggregates and determining what other pathways could be helpful in this regard.
“We are also further using the method we devised to visualize tau aggregates in human postmortem brain tissue slices from Alzheimer’s disease to determine the role of tau’s post-translational modifications in aggregation,” Lakadamyali said.
Perelman School of Medicine at the University of Pennsylvania

