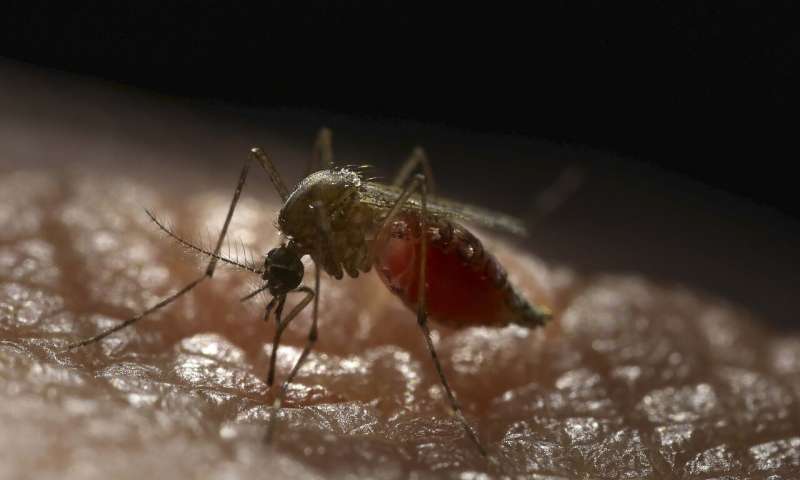
The annual death toll from malaria is over 400,000, with most of these deaths amongst children in sub-Saharan Africa. There has been little improvement noted in the last 5 years despite the large amounts of funding allocated to bed nets, insecticide spraying and antimalarial drugs. An efficacious vaccine is needed to try and reach the WHO goal of reducing malaria deaths by at least 90% by 2030.
R21/Matrix-M, a malaria vaccine developed at the Jenner Institute, University of Oxford, showed efficacy of 77% over 12 months in a recently reported phase IIb trial. First vaccinations have now begun in Mali in a larger phase III trial which is hoped to lead to licensure of this malaria vaccine by 2023. This phase III trial will assess efficacy and safety in 4800 children across five sites in Burkina Faso, Kenya, Mali and Tanzania. This is a double-blind, randomised, controlled trial where participants, aged 5-36 months, will receive three vaccinations 4 weeks apart and a booster vaccination 1 year later. The vaccine is being assessed in areas of differing malaria transmission and seasonality.
The University of Oxford has partnered with Serum Institute of India Pvt Ltd. (SIIPL) for the manufacturing of R21/Matrix-M to ensure provision of low high volumes of low-cost vaccine, and access in countries where it is required the most. SIIPL has confirmed its commitment to the provision of >200 million doses per year after licensure, which will be adequate supply for children most at risk of malaria in sub-Saharan Africa.
Professor Adrian Hill, Director of the Jenner Institute, University of Oxford said: “The start of a phase III licensure trial is always an import milestone in the development of a vaccine. This large malaria trial is the culmination of many years of laboratory research and assessment of numerous candidate vaccines in early-stage clinical trials with large numbers of collaborators.”
Professor Abdoulaye Djimdé, Director of the Malaria Research and Training Centre—Parasito (MRTC-P), University of Science, Techniques and Technologies of Bamako (USTTB), said: “We are thrilled to be the first site to enroll volunteers in the Phase 3 trial of this very promising R21 vaccine. We will utilize our more than two decades of experience in malaria vaccine testing towards successful completion of this trial”
Professor Halidou Tinto, Principal Investigator of the Nanoro, Burkina Faso trial site, said: “This is a very important moment in the development of the R21 malaria vaccine candidate. We hope that the public-private partnership behind this pivotal Phase III trial will confirm the high efficacy and good safety profile seen in our phase II trial in Nanoro. The five African institutions involved in this partnership have here a historic role to play. We are all committed to work hard in order to generate data that will provide regulators and policy makers with the evidence needed to support the registration of this vaccine. If successful, this vaccine should be made available as quickly as possible to complement existing malaria prevention tools”
Professor Jean Bosco Ouedraogo, Principal Investigator at The Institute of Sciences and Techniques in Bobo-Dioulasso, Burkina Faso said: “The R21 vaccine is a promising malaria prevention tool to help accelerate malaria elimination, particularly in high burden countries in Africa. The Phase II trial demonstrated high vaccine efficacy in children and I’m proud that it was done in Burkina Faso. I am really happy to be part of this key new trial to evaluate the vaccine’s safety and efficacy in an area of perennial transmission near Bobo-Dioulasso.”
University of Oxford