In 2015, researchers at UC San Francisco found a structure inside of tumor cells that biologists had never seen before. Even more surprising, a closer examination of the structure revealed that it contained signaling proteins known as receptor tyrosine kinases, or RTKs, which were thought to reside exclusively on the cell’s surface. The researchers reported the existence of the novel structures, but their exact identity and function remained elusive.
Now, in a paper published in the journal Cell, the same UCSF research team has figured out precisely what these structures are, how they form and their role in cancer. The findings represent a novel mechanism that tumors can use to promote their growth and survival, but they also reveal an Achilles’ heel that may lead to a new class of cancer-fighting drugs.
“This is among the more exciting discoveries that the lab has made,” said Trever Bivona, MD, Ph.D., professor of medicine and senior author of the study. “It opens up a whole new way of thinking about how receptor tyrosine kinases perform their oncogenic signaling function and may offer a new way to address drug resistance in cancer.”
Mutant RTKs transform healthy cells into cancer
In healthy cells, RTKs are embedded in the lipid membranes that surround most cellular compartments, most notably the surface of the cell itself. Though RTKs produce signals that promote cell division and growth, the cell is usually able to exercise tight control over RTK activity by switching these signaling proteins on and off. But this process can become dysregulated and lead to cancer.
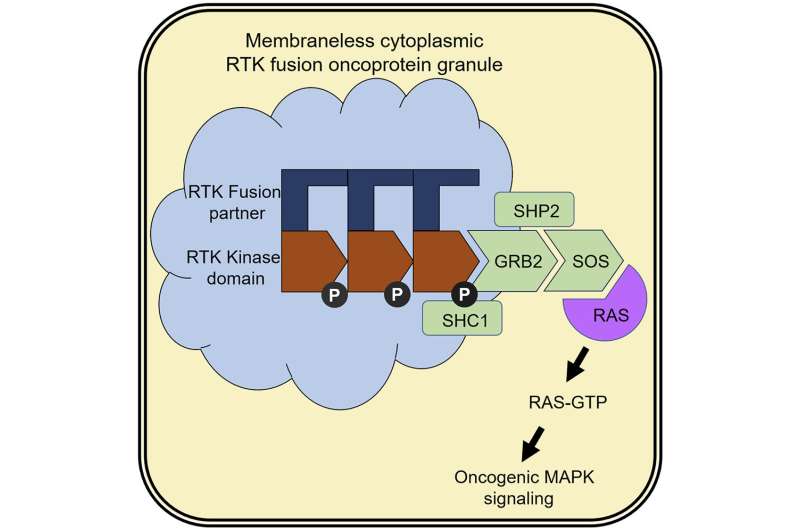
For reasons not yet fully understood, the genome can sometimes become unstable, causing the genes that encode RTKs to undergo a kind of genetic mix-and-match. When this happens, segments of RTK genes fuse with fragments of other genes to form mutant genes that encode what are known as “fusion proteins.” These fusion proteins retain the segments of RTK that tell the cell to grow and divide, but they’re missing key portions that allow the cell to switch them off. As a result, RTK fusion proteins can trigger the unfettered cell growth that’s a hallmark of cancer.
But the off switch isn’t the only thing that these fusion proteins are missing. They’re also missing the “molecular GPS” that normally directs an RTK to insert itself into a lipid membrane. Though scientists have long known that this membrane-targeting function was lost during the formation of RTK fusion proteins, they didn’t understand where these mutant proteins went or how they functioned if they couldn’t embed themselves in a membrane. The new study has shed light on this long-standing mystery.
Fusion proteins assemble into novel structures that promote tumor growth
The scientists found that fusion proteins are able to accumulate in the cell’s cytoplasm—the gel-like liquid inside of cells—and form the novel structures, which consist primarily of mutant RTKs. In contrast with most cellular compartments, which are surrounded by a lipid membrane, these structures are notable for having no membrane at all.
“They’re basically assemblies of proteins sitting in cytoplasm,” Bivona said. Though they lack the complexity of most cellular compartments, the structures are anything but simple, and they’re certainly not benign.
The researchers found that these structures could recruit and activate other signaling proteins that are responsible for transforming healthy cells into cancer. In short, when these structures form, they become factories that produce cancer-promoting signals, including activation of molecular switches known as RAS GTPases that, when turned “on” by these mutant RTKs in the cytoplasm, promote cancer.
“This is the first demonstration that an oncogenic kinase can form these kinds of structures, and also the first demonstration of RTKs becoming activated and signaling to RAS at a location without a lipid membrane,” said Bivona.
Though the researchers were able to show how these structures contribute to cancer, they also identified a weak spot, which may offer hope for an entirely new kind of cancer therapy.
Structure’s Achilles’ heel may lead to new cancer-fighting drugs
Though RTK fusion proteins have long been a target of anti-cancer drugs, these drugs have generally worked by blocking the site in the fusion protein responsible for producing pro-cancer signals. Though these therapies tend to be effective at first, most cancers mutate, eventually rendering these drugs ineffective. The new study, however, suggests that an alternative approach that prevents fusion protein structures from forming may offer new hope for patients with RTK-driven cancers.
The study shows that RTK fusion proteins must assemble into larger structures in order to stimulate tumor growth and survival. The researchers found that they could prevent these structures from forming by mutating specific parts of the RTK fusion protein. They were also able to identify a handful of proteins often associated with RTK signaling that, when removed from the cell, also prevented the structures from forming. Importantly, the scientists found that RTK fusion proteins were unable to produce cancer-promoting signals unless they were part of the larger structure.
The researchers believe that this requirement represents an Achilles’ heel that may inspire a new class of targeted therapies that work by disrupting the formation of these structures. But according to Bivona, a lot of work remains to be done to understand what rules control the assembly and degradation of these structures, and how we can exploit this knowledge to develop new therapies.
Jason Alvarez, University of California, San Francisco

