
A line in this week’s federal budget allocating A$288.5 million to repetitive transcranial magnetic stimulation (rTMS) therapy might pass most people by.
This is a brain stimulation technique that’s been used to treat conditions such as depression for almost ten years in Australia, but which has not been funded through Medicare and so has had very limited availability.
Soon, it will be available on the Medicare Benefits Schedule for people with depression that hasn’t responded to other treatments, funding I’ve led applications for since 2012, and treatment I provide.
While we know rTMS can work, and is generally safe, we’re not entirely sure how it works. Here’s what the evidence says so far.
What is it?
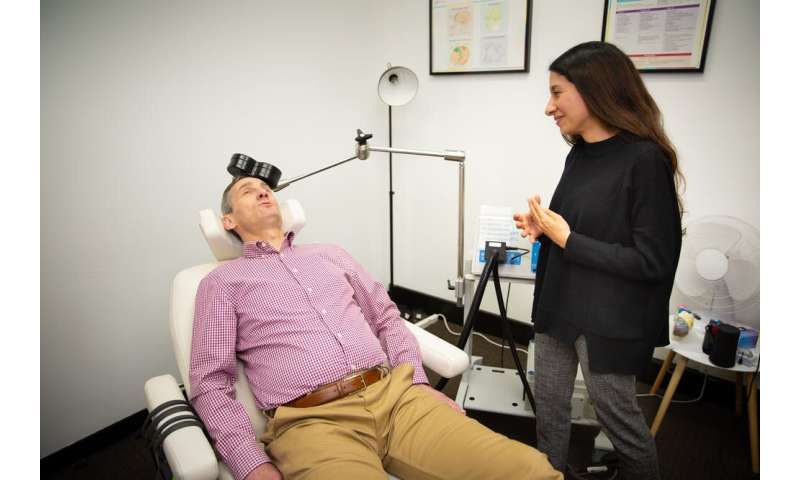
In rTMS, a machine produces and applies a highly targeted, pulsed magnetic field to a specific area of the brain, towards the front, known as the prefrontal cortex. This is an area we believe isn’t working normally in people with depression.
During treatment, an electrical current passes through an electromagnetic coil held near the scalp to stimulate the nerve cells.
The person sits in a comfortable chair, awake and alert during treatment. It’s quite different from electroconvulsive therapy (ECT, the modern version of shock treatment). Unlike ECT, rTMS does not involve producing a seizure and does not require the person to be asleep and under an anesthetic.
How does it work?
We know repeated rTMS stimulation, over the course of weeks, increases nerve activity in the area under the coil. It also changes the strength of connections between different areas of the brain. This is thought to help restore the normal interaction between brain regions, although these ideas are still theoretical and definitely not proven.
Antidepressant medications may act in similar ways, but less directly. The chemicals they affect can influence brain function quite widely: tuning activity or connectivity in brain circuits up or down. rTMS probably does this more directly. By directly making nerve cells fire we can directly change their activity levels. These more direct actions could possibly explain why rTMS may work in some people who have not responded to medication.
Trials show rTMS treatments result in a gradual improvement in depression. A person’s mood will slowly lift, usually over the course of several weeks, they will become more interested in things, sleep better, be more motivated and have more energy.
In people who respond, depression can go away for several months up to many years. If depression returns, most people will get better again with further treatment.
Does it work? Is it safe?
Evidence collected over the past 25 years and collated shows rTMS is a safe and effective treatment for people with treatment-resistant depression. These are the 30-40% of people diagnosed with depression who have tried antidepressant medications, usually two or more, and haven’t seen any or sufficient relief. They have persistent, ongoing depression with major effects on their ability to function, work and lead normal family lives.
The treatment is usually well-tolerated. Although some people experience a strong tapping sensation on the scalp, scalp pain during treatment, or a headache afterwards.
Some 25 years of research have failed to identify any long-term negative consequences. People are much more likely to experience significant side-effects with antidepressant medications than with rTMS.
Studies have also compared the effectiveness of rTMS with other treatments, such as different medications. This study, written by authors from the pharmaceutical industry, only reports the benefits of medications in the study abstract but rTMS was clearly the superior intervention on outcomes across the full analysis.
Finally, research including more than 5,000 people having the treatment shows it provides meaningful and valuable clinical benefits in the real world, outside clinical trials.
This treatment isn’t perfect
Like many medical treatments, rTMS is not perfect. We are trying to develop ways to improve outcomes by better individualizing the treatment. For example, we are trying to better understand the exact spot to target in the brain and how to match the frequency of stimulation to an individual person’s pattern of brain activity.
We’re also trying to get around one of the biggest issues: its relative inefficiency.
A course of rTMS typically involves going to a clinic for a 30-minute treatment session, five days a week, for up to six weeks, which is time-consuming and requires a significant commitment. We are working to make the application less time-consuming and potentially shorten the duration of therapy.
One of the most significant implications of the government funding of rTMS therapy through Medicare is that it will become more widely available, including in outer suburban and rural areas.
The funding will take some months to be implemented but once available will be accessible by a referral from a GP or psychiatrist.
Paul Fitzgerald, The Conversation

