In recent years, immunotherapy has revolutionized the field of cancer treatment. However, inflammatory reactions in healthy tissues frequently trigger side effects that can be serious and lead to the permanent discontinuation of treatment. This toxicity is still poorly understood and is a major obstacle to the use of immunotherapy. Scientists from the University of Geneva (UNIGE), Switzerland, and Harvard Medical School have succeeded in establishing the differences between deleterious immune reactions and those targeting tumor cells. While the immune mechanisms are similar, the cell populations involved are different. This work, published in the journal Science Immunology, could lead to better targeted, more effective, and less dangerous treatments for cancer patients.
Based on massive stimulation of the patient’s immune system, immunotherapies have saved many lives. Unfortunately, they are not without consequences. “When the immune system is activated so intensively, the resulting inflammatory reaction can have harmful effects and sometimes cause significant damage to healthy tissue,” says Mikaël Pittet, holder of the ISREC Foundation Chair in Onco-Immunology at UNIGE Faculty of Medicine Department of Pathology and Immunology and Centre for Translational Research in Onco-Haematology, and a member of the Swiss Cancer Centre Leman. “Therefore, we wanted to know if there are differences between a desired immune response, which aims to eliminate cancer, and an unwanted response, which can affect healthy tissue. The identification of distinctive elements between these two immune reactions would indeed allow the development of new, more effective and less toxic therapeutic approaches.”
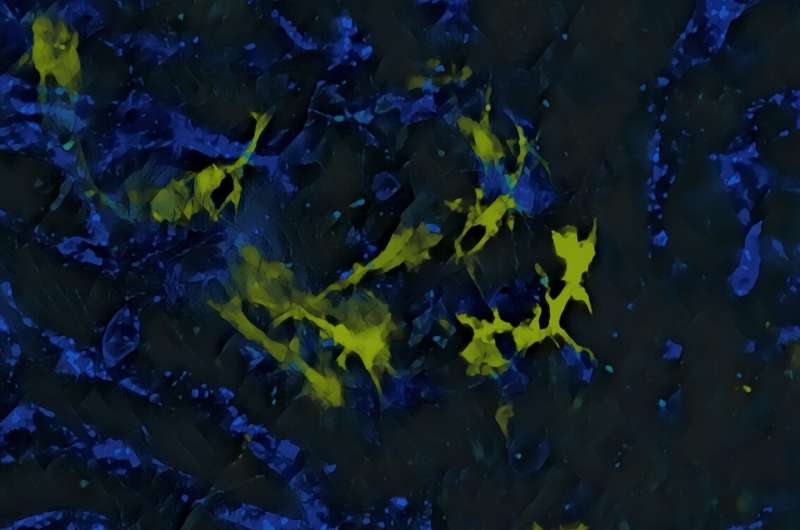
Using liver biopsy samples from patients treated at the CHUV and the HUG who had suffered such toxic reactions, the scientists studied the cellular and molecular mechanisms at work to reveal similarities and dissimilarities.
A similar response, but with different cells
In an immunotherapy-related toxic response, two types of immune cells—macrophage and neutrophil populations—appear to be responsible for attacking healthy tissue, but are not involved in killing cancer cells. In contrast, another cell type—a population of dendritic cells—is not involved in attacking healthy tissue but is essential for eliminating cancer cells.
“Immunotherapies can trigger the production of specialized proteins that alert the immune system and trigger an inflammatory response, explains Pittet. In a tumor, these proteins are welcome because they allow the immune system to destroy cancerous cells. In healthy tissue, however, the presence of these same proteins can lead to the destruction of healthy cells. The fact that these inflammatory proteins are produced by such different cells in tumors and healthy tissue is therefore an interesting finding.”
Dendritic cells are very rare, whereas macrophages and neutrophils are much more common. Some macrophages are present in most of our organs from embryonic development stages and remain there throughout our lives. Contrary to previous belief, these macrophages do not necessarily inhibit inflammation, but when stimulated by immunotherapies, they can trigger a harmful inflammatory response in the healthy tissue where they reside, thus explaining why toxicity can affect different organs.
Neutralizing neutrophils for a double benefit
When macrophages are activated by drugs, they produce inflammatory proteins. These in turn activate neutrophils, which execute the toxic reaction. “This opens the possibility of limiting immunotherapy’s side effects by manipulating neutrophils,” says Pittet.
The research team confirmed their discovery by studying the immune reactions of mice whose cell activity was modulated with genetic tools. They were able to identify a loophole that could be exploited to eliminate these side effects. Indeed, neutrophils produce some factors that are important for the development of toxicity, including TNF-α, which could be a therapeutic target. TNF-α inhibitors are already used to modulate the immune response in people with arthritis and could perhaps be useful in the cancer setting to inhibit the toxic effects of neutrophils during immunotherapy. “Furthermore, inhibiting neutrophils could be a more effective way to fight cancer: in addition to triggering a toxic response, some of these cells also promote tumor growth. Thus, by managing to control them, we could have a double beneficial effect: overcome the toxicity in healthy tissues, and limit the growth of cancerous cells,” says Pittet.
University of Geneva

