New research from the Australian Regenerative Medicine Institute (ARMI) at Monash University has identified novel cell types and RNA signals that may assist with brain and spinal cord repair.
Tissues and organs have different capacities to regenerate after injury or disease. Identifying cell types and signals that can promote repair is particularly important for organs that repair poorly such as the brain and spinal cord.
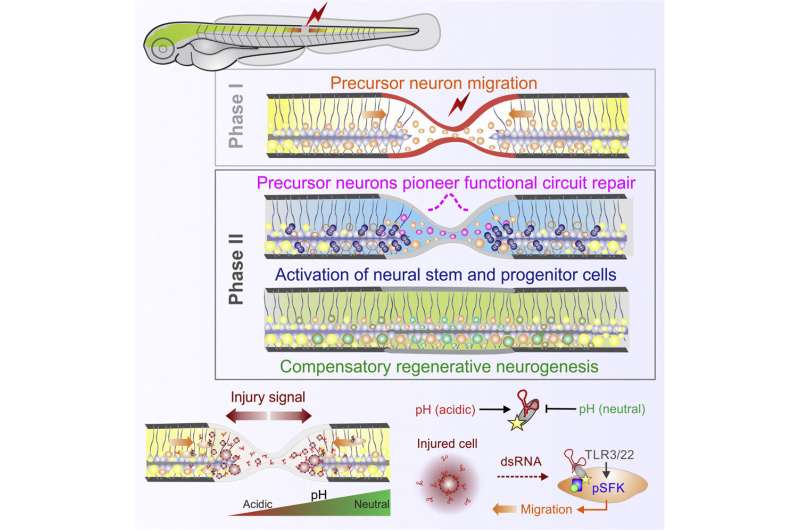
Using zebrafish models, researchers identified ribonucleic acid (RNA) as an injury-induced damage signal that triggers recruitment of neurons and neural tissue repair, mobilizing a previously unknown brain neuron reservoir that is in standby mode for repair which has implications for aging and degenerative diseases.
The findings, published in Developmental Cell, showed that blocking or enhancing the recruitment of these immature neurons halted or sped up circuit and functional recovery, respectively, demonstrating the power of these cells in boosting neural repair.
The lead author, Associate Professor Jan Kaslin says: “Brain and spinal cord injuries are devastating events that have a life-long impact on the patients’ life with wide-reaching socio-economic effects. At present, there are no effective treatments or strategies to improve healing of the nervous system.”
“Modulating RNA-mediated inflammation using drugs is a tractable therapeutic avenue that could be used to restrict damage and improve tissue regeneration.”
Associate Professor Kaslin says the role of dormant neurons merits further research as these neurons on standby mode represent an untapped cellular reserve that previously has received little attention. This reserve of neurons may play a pivotal role during aging, disease and repair.
He adds, understanding that the RNA released from injured cells can act as an early damage signal to initiate regenerative programs in the nervous system is important as they can be harnessed for healing.
“RNA-induced inflammation and migration of precursor neurons initiates neuronal circuit regeneration in zebrafish” is published in Developmental Cell.
Monash University

