Researchers from Duke-NUS Medical School, together with collaborators from the National Centre for Infectious Diseases (NCID) and Singapore General Hospital (SGH), have discovered a simple and rapid method to measure the T-cell immune response to the SARS-CoV-2 virus, which causes COVID-19.
A growing body of data now demonstrates the importance of both T cells and antibodies in the coordinated immune response against SARS-CoV-2. This method is a further boost to scientists who seek to routinely monitor and assess SARS-CoV-2-specific T-cell responses in vaccinated or convalescent individuals, as well as to test and verify the effectiveness of vaccines.
“T cells play a vital role alongside antibodies in protecting people against COVID-19, but they are much harder to detect and measure,” said Dr. Anthony Tanoto Tan, Senior Research Fellow with Duke-NUS’ Emerging Infectious Diseases (EID) Program and first author of the study. “Our research offers a feasible approach that can overcome the current limitations faced in detecting spike-specific T-cell responses, and will help better evaluate the protective role played by T cells in our immune system.”
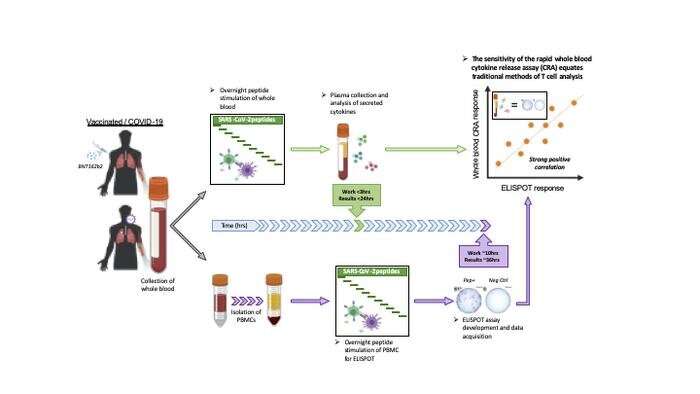
For the study, published in the Journal of Clinical Investigation, scientists took blood samples from volunteers who were vaccinated against COVID-19, or who had been infected and then recovered from the disease. They then introduced small fragments of the SARS-CoV-2 spike protein directly into the blood samples. In response to these fragments, the T cells released chemical signals called cytokines, which are much easier to detect and measure than T cells, and are already being tracked to monitor T-cell activity for the diagnosis of diseases such as tuberculosis.
Building on that, the team showed that the test, called Cytokine Release Assay (CRA), can reliably identify and quantify specific T cells present in the blood of people who have been vaccinated against COVID-19, or have recovered from SARS-CoV-2 infection. Working with different blood samples from more than 200 people, the researchers desmonstrated that the CRA test was as sensitive as existing methods used to find and measure T-cell activity.
“This discovery allows a rapid and large-scale expansion of studies to track T-cell activity across the world, while not requiring specialized or expensive equipment,” said Professor Antonio Bertoletti from Duke-NUS’ EID program, the study’s corresponding author. “The study results confirm that the level of antibodies against SARS-CoV-2 in blood samples does not always correlate with the T-cell response. With this rapid test, we can help define the correlates of protection from T cells and antibodies for the development of COVID-19 vaccines.”
Professor Patrick Casey, Senior Vice-Dean for Research at Duke-NUS, said, “This important study advances our understanding of the human body’s immune response at a critical juncture in this pandemic. As validated in this research, repurposing the well-established CRA test to fast-track the evaluation of T-cell responses in COVID-inoculated or -convalescent patients adds a new dimension to vaccine strategies as we battle the threat of new and emergent variants.”
To bring this discovery to market, Duke-NUS has licensed the assay to Hyris, an innovation-based biotechnology company, which will leverage its Hyris SystemTM to further develop this rapid SARS-CoV-2 T-cell test for clinical use globally.
Duke-NUS Medical School

