Based on previous studies of the COVID-19 pandemic, it is known that a specific form of white immune cells, natural killer (NK) cells, make an important contribution to the early antiviral immune response against SARS-CoV-2. An international team led by the University of Bonn has now found that in severe courses of COVID-19, the ability of natural killer cells to prevent pathological proliferation of fibrous tissue in the lungs is often impaired. Major parts of the study were conducted at the University Hospital Bonn and the German Center for Neurodegenerative Diseases (DZNE). The results have been published online in advance in the reputed journal Immunity. The print version will appear shortly.
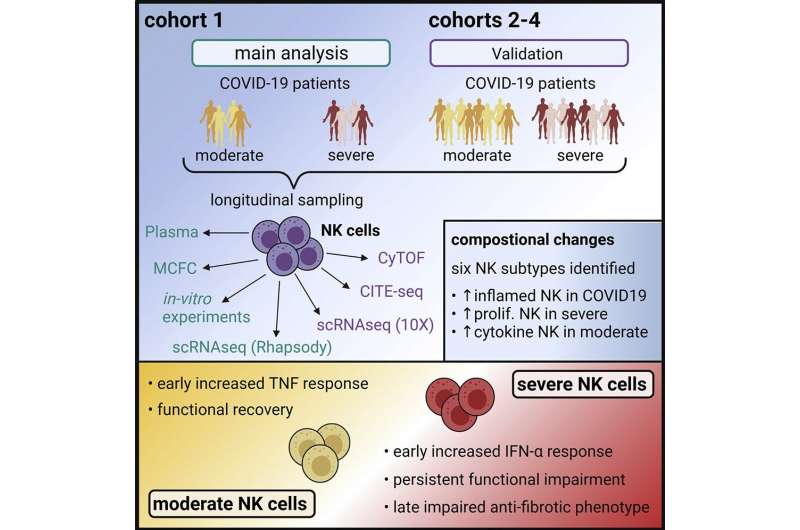
Until now, it was unclear to what extent Natural killer cells contribute to the development of severe COVID-19 infection. Scientists of the University of Bonn and the German Center for Neurodegenerative Diseases (DZNE), together with an international team, have now been able to investigate in detail the role of NK cells in the course of COVID-19 in a multicenter study.
Persistent dysfunction in severe COVID-19 courses
“Already in the very early stage of severe disease, NK cells display a specific molecular fingerprint that is attributable to the so-called type I interferons. This is accompanied by a significant dysfunction that persists for several weeks,” says Prof. Dr. Jacob Nattermann, study leader and Deputy Director of the Department of Internal Medicine I of the University Hospital Bonn.
The researchers examined blood samples from 205 subjects between the 1st and 6th week after symptom onset. This allowed them to classify the molecular properties and functions of the cells over time.
“NK cells from COVID-19 patients with moderate symptoms also showed an impaired function at the beginning of the disease, but this normalized after a short time,” reports Prof. Dr. Joachim Schultze, co-leader of the study and Director of Systems Medicine at the DZNE.
NK cells lose antifibrotic activity
Since severe COVID-19 infection is usually accompanied by tissue remodeling in the lung (pulmonary fibrosis) and NK cells are known for their antifibrotic properties, this aspect was also investigated. “Three weeks after infection, molecular patterns resembling those observed in other immune cells in the context of fibrosis formation were evident in NK cells in severe courses. In line with these observations, these NK cells have significantly lost their antifibrotic capacity, which may have an impact in fibrotic remodeling of the lung,” added Dr. Anna Aschenbrenner, also co-leader of the study and working at the Life & Medical Sciences (LIMES) Institute of the University of Bonn and at DZNE.
“In summary, our data provide a detailed insight into the role of NK cells in the immunopathogenesis of COVID-19. Future study will have to show whether this may aid the development of novel therapeutic approaches,” said Dr. Benjamin Krämer, one of the first authors from the Department of Internal Medicine I of the University Hospital Bonn.
“The COVID-19 emergency taught us that collaborating with some of the best groups in Germany and around the world allows us to achieve clinically important results at an unprecedented pace. We will continue to work in this way on NK cells, but also on other cell types,” comment co-first authors Rainer Knoll and Dr. Lorenzo Bonaguro, both from DZNE. “This will hopefully also allow us to understand how these different cells interact in severe COVID-19 and allow us to fully understand this complicated disease,” say co-first authors Michael ToVinh and Jan Raabe, both Ph.D. students at the Department of Internal Medicine I of the University Hospital Bonn.
University of Bonn

