An especially deadly subtype of T-cell lymphoma is distinguished by unique mutations in a specific protein signaling pathway, according to a study published in the journal Blood.
Correcting the downstream effect of these mutations with a pharmacological inhibitor could prove a worthwhile treatment and further showcase the benefits of precision medicine, according to Jaehyuk Choi, MD, Ph.D., the Ruth K. Freinkel, MD, Research Professor and senior author of the study.
“All patients are different, but we tend to treat them all the same,” said Choi, who is also an associate professor of Dermatology and of Biochemistry and Molecular Genetics. “Our goal is precision medicine. This means we will develop simple molecular tests to identify the best way to treat individual patients.”
Cytotoxic cutaneous T-cell lymphoma (CTCL) is a rare but highly aggressive skin cancer. Previous studies have found mutations in the pathway known as JAK-STAT in other blood cancers, but that pathways’ association with CTCL was unknown.
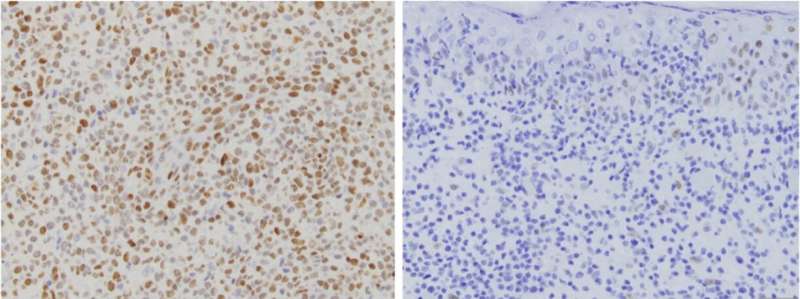
In the current study, Choi and his collaborators performed genetic sequencing on archived biopsy samples of CD8+ aggressive CTCL, searching for mutations in the JAK-STAT. The investigators discovered several gene fusion events: whole chromosomes interacting with other chromosomes, all involving JAK2, a kinase in the pathway.
“These events are rare to begin with, so the fact that they all associate with JAK2 is astonishing,” said Katie Lee, a research fellow in the Choi laboratory, a medical student at the University of Illinois-Chicago and lead author of the study.
Further, these JAK2 mutations were nearly perfectly predictive of the aggressive CD8+ subtype, suggesting that the presence of those mutations could serve as a useful biomarker.
“Pathologists can see subtle differences between cancer subtypes under the microscope, and now we can link these differences directly to molecular aberration,” Choi said.
JAK2 mutations improve cancer proliferation but these downstream effects could be countered with a JAK2 inhibitor, and a clinical trial utilizing this strategy is already in progress.
“The molecular features of the mutation are as such that we have a very good reason to predict complete responses to this therapy,” Choi said. “We think this agent can offer a chance for a cure.”
Will Doss, Northwestern University

