Vaccines have been a mainstay in modern medicine since the late 18th Century, but there is evidence that societies have been using them for more than a thousand years. Throughout this time, all vaccines have operated by activating various immune cells against an infection. A new study in Science Advances led by ASHBi Professor Hideki Ueno reports how the transcription factor Tox2 controls the activation of one of these cells, TFH cells.
One of the most effective ways by which the body fights off an infection is by producing antibodies. Antibodies are not easy to make in the lab, but vaccines stimulate antibody production in the body for a specific infection, such as SARS-CoV-2, the virus responsible for the COVID-19 pandemic.
A number of immune cells are involved in the antibody production, including TFH cells, or T follicular helper cells.
“TFH cells are essential for producing high-affinity antibodies and for generating long-lived antibody-producing cells,” said Ueno. In other words, although TFH cells themselves do not make antibodies, their function heavily influences the immune cells that do. In fact, a number of antibody disorders are associated with their dysfunction.
Reaffirming the importance these cells have in combating infection, or adaptive immunity, is their presence in blood even decades after a vaccination, evidence of them having immunological memory, which describes the ability to promptly react when encountering a pathogen for the second time. Understanding the molecules that enable this persistence, argues Ueno, will benefit vaccine research.
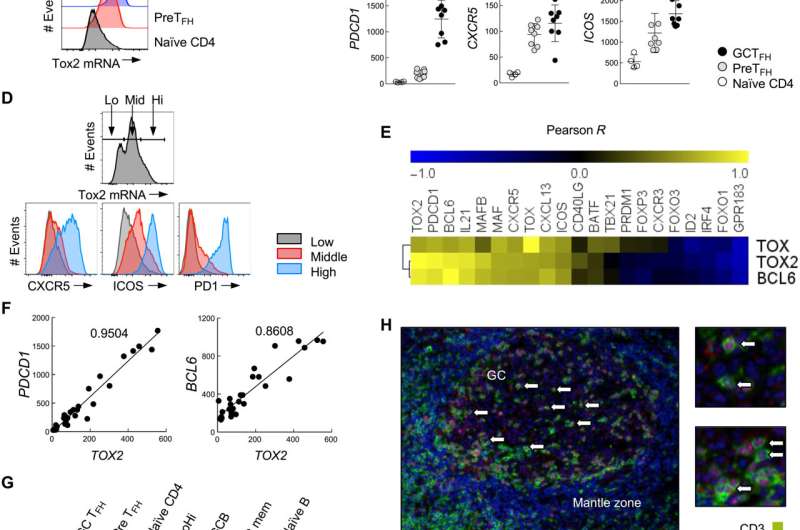
“The molecular mechanism associated with the sustained TFH cell response is unclear. We found that the expression of Tox2 is important,” he said.
Tox2 is recognized for its contribution in immune cell exhaustion; immune cell exhaustion is a discovery that led to Kyoto University Professor Tasaku Honjo winning the Nobel prize in 2018. Cell exhaustion is a natural effect that prevents autoimmune diseases from occurring, but also allows cancers to persist.
The new study found, however, that Tox2 has a very different effect in TFH cells.
“Our study shows that Tox2 is important for the long-term survival and functional maintenance of TFH cells. This suggests a role distinct from other T cells,” said Ueno.
To test this hypothesis, Ueno and his colleagues did two experiments. In one, they overexpressed Tox2 in human TFH cells. In the other, they prevented its expression in mice.
Normally, human TFH cells transform into other types of immune cells upon an immune stimulation. However, the human TFH cells overexpressing Tox2 were stable.
“TFH cells will spontaneously transform with TCR stimulation because they are not terminally differentiated,” said Ueno. “We found that overexpressing Tox2 insulated the cells from this spontaneous transformation. This finding indicates Tox 2 is important for the persistence of TFH cells.”
In contrast, mice with deficient Tox2 showed low levels of TFH cells in the blood and poor antibody responses to infection.
The experiments suggest that targeting Tox2 could be used therapeutically to modulate TFH cells.
“Our findings show the importance of Tox2 for the maintenance of TFH cells and establishing TFH cell memory. This will provide important insights for the development of vaccines that induce potent and durable antibody responses,” said Ueno.
Kyoto University

