A molecule known as anaplastic lymphoma kinase (ALK) is a driver of several cancers, including pediatric neuroblastoma, B-cell lymphomas, and myofibroblast tumors. But for years much about this molecule—its role in the body, which molecules interact with it, what it looks like—has remained unknown, limiting efforts to target it for treatment.
Now, two Yale-led studies published Nov. 24 in Nature reveal the structure of this molecule, opening new paths for cancer treatment development.
The ALK molecule is a receptor—a protein that sits in the membrane of a cell and responds to other molecules that bind to it—found primarily in the brain and central nervous system. For decades it was described as an “orphan receptor” because its ligand—or the substance that binds to and activates it—was unknown until Yale researchers resolved that mystery in 2015. That discovery was an important step toward understanding this receptor and the role it plays in the human body.
Uncovering its structure represents another leap forward, researchers say.
“To target a receptor like this for treatment, you would like to know how to block it or stimulate it, but you cannot do this unless you know the structure,” said Joseph Schlessinger, the William H. Prusoff Professor of Pharmacology and chair of Yale’s Department of Pharmacology.
Receptors are one of the ways our bodies send signals, or messages, to specific cells or tissues. They respond to specific substances, and once those substances attach to the receptor, they cause some sort of action—the receptor can be activated or deactivated, and that action will have an impact on the cell on which the receptor sits.
Daryl Klein, an assistant professor of pharmacology, is the senior author of the first of the two new studies. In the study, he and his colleagues at the Yale Cancer Biology Institute used X-ray crystallography to visualize the part of ALK that binds to other molecules, allowing them to see its structure.
And it turns out the structure is very unusual—a key part had never been seen before in a human protein. “It really, truly, was surprising,” said Klein.
The unexpected region is made up of chains of glycine, which is an amino acid, or a building block of protein. The region is an extremely rigid and stable part of the receptor and plays a key role in the overall positioning of ALK within the cell membrane. Klein says mutations in this part of the receptor are linked to cancer, and understanding what these mutations do to the structure of the receptor and its function will be essential for understanding how they lead to cancer.
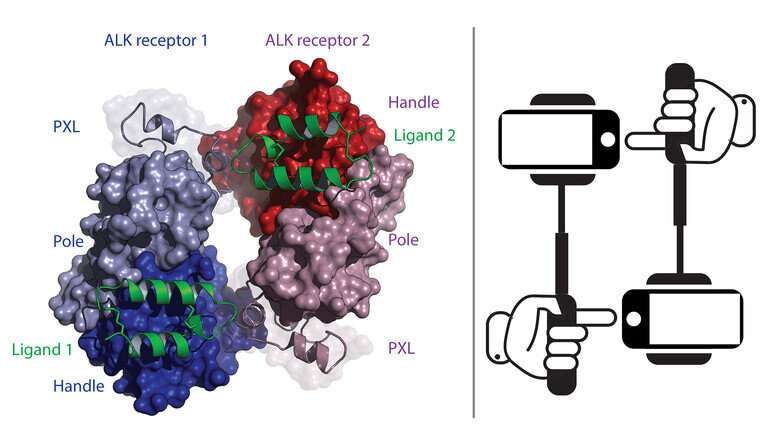
Klein’s research team, led by postdoctoral researcher Tongqing Li and senior research scientist Steven Stayrook, were able to observe how ALK activates after binding to its ligand. Specifically, they found that two ligand-bound ALK receptors come together, with each grabbing on to the opposite side of the other. This joining of two molecules is known as a dimer, and once the two ALK molecules form a dimer, the receptors are activated.
That discovery, Klein said, led researchers to their next question: “How can we deactivate the receptor when it’s on but shouldn’t be, like when cancer develops?”
Using two antibodies known to interact with ALK, Klein and his team were able to do just that, observing how the antibodies—provided by the New Haven-based company Celldex Therapeutics—were able to disrupt ALK’s function. One prevented the ligand from binding to ALK while the other prevented the two ALK receptors from coming together and activating.
This information will offer powerful insights for developing cancer treatments, Klein said. In fact, his team is working to leverage this structure to create new therapeutics. “We now know exactly where we want to target to prevent the activation,” he said.
A separate study co-led by Schlessinger’s lab and researchers at St. Jude Children’s Research Hospital used additional methods to assess ALK’s structure. Through X-ray crystallography, cryogenic electron microscopy, and nuclear magnetic resonance spectroscopy, the researchers uncovered the atomic details of ALK and how it is activated by its natural ligands.
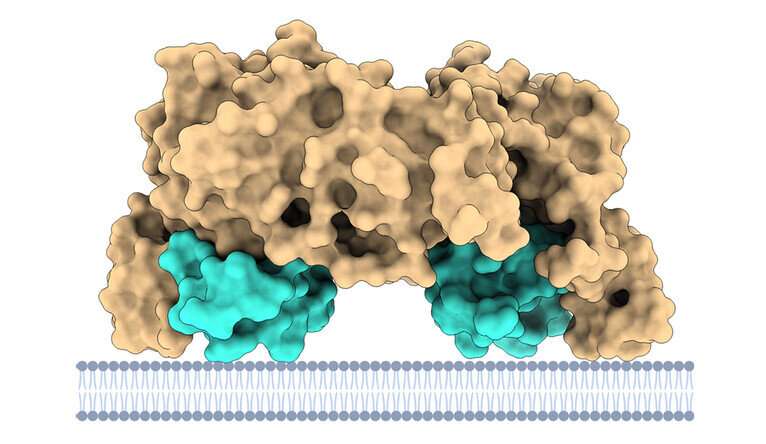
Through these techniques, the team drew the same conclusions as Klein’s—ALK has an unusual structure with unique features not seen in other receptors of the same family. Even its positioning within the cell membrane stands out from other receptors.
“The receptor lies on the cell surface. It’s not standing up perpendicularly to the cell like most receptors,” said Schlessinger.
This positioning, he said, causes the ligand to interact with the receptor and the cell surface at the same time. “This has never been seen before in any ligand-receptor interactions,” he said.
With this understanding of ALK’s structure, Schlessinger is now working on another piece of the puzzle—its ligand. “We know nothing about how it works,” he said. “But like its receptor, it could also play a role in cancer. It might also play a role in metabolic diseases and how these processes are connected.”
In addition to developing treatment, Klein’s team is also working on understanding how mutations in ALK lead to cancer. And they’re tackling a question that led him to study ALK in the first place—why its ligand is not the same in vertebrates and invertebrates.
“Why would there be different ligands in invertebrates and vertebrates while the receptor itself is largely the same? There must have been enormous evolutionary pressure to start over from scratch in vertebrates—I think that’s where a lot of the story is going to be,” said Klein. “The answer will reveal something quite interesting.”
Mallory Locklear, Yale University

