Research led by CNIO scientist Héctor Peinado shows that the NGFR molecule drives the entire process of early metastasis in melanoma and that blocking it drastically reduces metastasis in animal models. The reduction was achieved using THX-B. This molecule is being tested for the treatment of other pathologies, which will accelerate its possible use in the treatment of tumors.
“We must not only look inside the tumor but also outside of it,” says Héctor Peinado, a researcher at the Spanish National Cancer Research Centre (CNIO). How tumors manipulate their environment to advance is one of the big questions that Peinado has been trying to answer for years. For decades “to fight tumors, researchers focused on studying their intrinsic behavior, but not on their surroundings.”
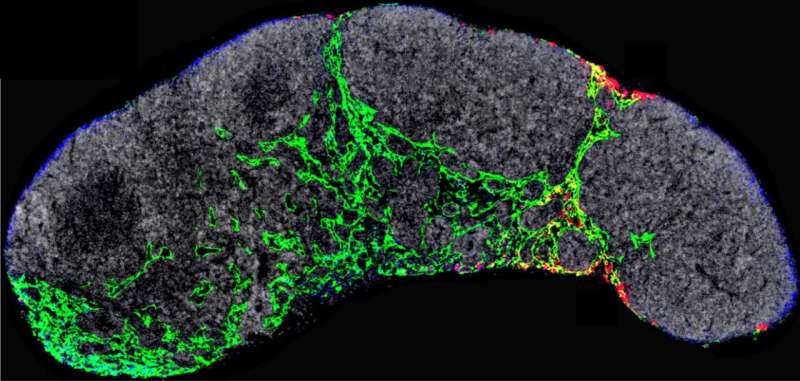
Peinado is the head of the CNIO’s Microenvironment & Metastasis Group, which studies the mechanisms involved in metastatic progression, including how nanoparticles called exosomes, which are released by tumors, manipulate the tumor microenvironment to favor metastasis. A paper published this week in the journal Nature Cancer describes how this critical process for melanoma progression occurs. Exosomes travel to the sentinel lymph node—the lymph node where metastasis initially occurs—from where they remotely prepare a favorable environment—the pre-metastatic niche—for metastasis. In this study, the researchers observed that the NGFR molecule drives this entire process.
The scientists also propose NGFR as a new biomarker of early melanoma metastasis to define risk groups and predict metastasis. “A higher number of NGFR-expressing metastatic cells in the sentinel lymph node correlates with a worse disease prognosis,” says Susana Garcia Silva, co-first author of the study.
Unlike other skin cancers, melanoma is one of the most aggressive tumors; it can metastasise when the primary lesion is still very small. There are no early disease markers or disease prediction markers, so discovering early and accurate methods for diagnoses using the NGFR biomarker can improve the prognosis of patients.
Pre-empting metastasis
Metastasis is the cause of 90% of cancer deaths. In most cases, they are detected too late. “If we can identify when a tumor is going to metastasise, even before it happens, during soil preparation, it will be easier to treat it and to contain it,” says Peinado.
Although exosomes—nanovesicles released by all cell types, including tumor cells—were discovered more than 30 years ago, they have not been widely studied until the last few years. In 2012, Peinado discovered in David Lyden’s lab in the U.S. how tumor cells release exosomes, which transfer biological information to the surrounding microenvironment to instruct it and promote metastasis even before the tumor cells themselves travel through the body.
“Until a few years ago, the microenvironment surrounding the tumors was overlooked. Now we know that the communication of tumors with their local environment and the rest of the organism is fundamental to understand cancer and its complications,” said Peinado in 2015, shortly after joining the CNIO to start his Microenvironment & Metastasis Group.
Melanoma cells, like many cells from other tumors, travel and spread through the body mainly via the blood circulation and the lymphatic system. These circulating tumor cells settle in the lymph nodes, which act as a reservoir or warehouse, and from there they carry out the changes for the formation of the pre-metastatic niche that will favor the colonization of other organs. “In this study, we focused on the mechanisms of what could be called the earliest stages of metastasis,” explains Peinado.
After seven years of extensive analysis, the researchers describe in Nature Cancer that exosomes released by melanoma cells are recruited by lymphatic endothelial cells in the lymph nodes. In these cells, the exosomes promote, via the NGFR molecule, further branching of the lymphatic vasculature and adhesion of tumor cells that will allow them to survive and migrate to other sites. “Melanoma cells secrete exosomes carrying NGFR to manipulate the behavior of lymphatic endothelial cells and facilitate metastasis.”
A possible first treatment to fight melanoma metastasis
“We knew that melanoma cells that initiate metastasis increase NGFR production, but nothing was known about a possible role of NGFR in exosomes and its effects outside the tumor.”
After discovering the role of this molecule in the early development of melanoma metastasis, the team decided to study the consequences of blocking it during tumor cell expansion in mice. To do this, they used a genetic approach, in which they eliminated NGFR from the exosomes, and a pharmacological approach, in which they used the NFGR inhibitor THX-B. In both cases, metastasis was drastically reduced, opening the way to a possible new treatment to combat metastasis.
This may become one of the first treatments to tackle metastasis in its earliest stages when it is most likely to be successful.
The inhibitor THX-B is being studied for the treatment of other diseases such as diabetic retinopathy, but its effectiveness in the treatment of cancer has not been explored. “We are currently developing its use for clinical application in patients.” These results may be extended to blocking metastasis in other types of tumors that overexpress NGFR.
The study also shows that the number of metastatic cells expressing NGFR in lymph nodes predicts disease progression in melanoma patients. “Analysis of these cells in the lymph nodes could serve as an important biomarker of disease progression and for early diagnosis,” says the researcher.
This research has been conducted with the international participation of Piotr Rutkowski (Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw), Michelle Levesque (University of Zurich Hospital), Uri Saragovi (McGill University, Montreal), Babak Mehrara (Memorial Sloan Kettering Cancer Center, New York) y David Lyden (Weill Cornell Medical College, New York), and national participation of Andrés Hidalgo (CNIC) and Molecular Cytogenetics Unit, Flow Cytometry Core Unit, Mouse Genome Editing Core Unit, Electron Microscopy Unit, Bioinformatics Unit, Proteomics Core Unit and Confocal Microscopy Core Unit at CNIO.
The Spanish National Cancer Research Centre

