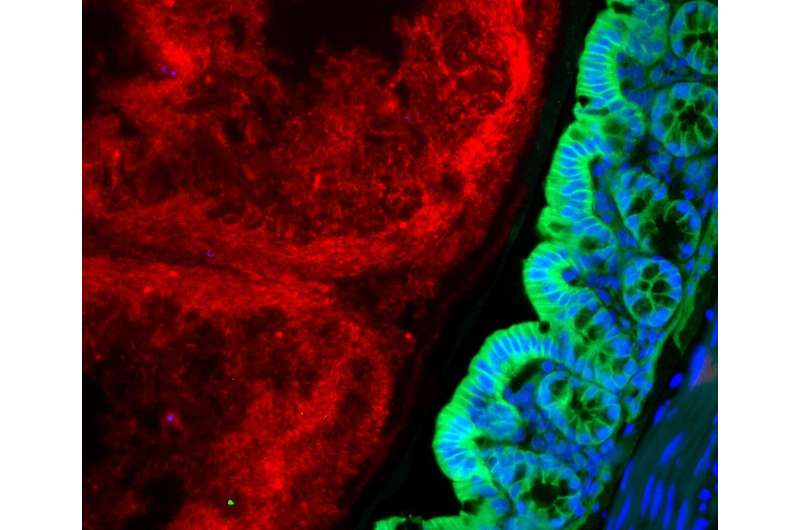
Around 500 to 1,000 different types of bacteria, fungi and other microorganisms colonize our intestines. All of them together form the intestinal microbiome. As we now know, these microbes play an important role in maintaining health. This is especially evident when the composition of the microbiome becomes unbalanced, as is the case in people with chronic inflammatory bowel diseases. Their intestinal microbiome contains fewer types of bacteria than that of healthy people. But so far, precisely how a modified microbiome contributes to the development of diseases is largely unknown.
PD Dr. Felix Sommer and his team from the Cluster of Excellence Precision Medicine in Chronic Inflammation (PMI) are trying to better understand the complex interactions between the host and their microbiome. While doing so, they encountered an enzyme called hexokinase 2 (HK2). The HK2 enzyme is produced in greater quantities in the event of inflammation and is regulated by the microbiome. “We were able to show in the animal model that by administering the short-chain fatty acid butyrate, the HK2 levels in intestinal epithelial cells were lowered and inflammation was ameliorated. The protective effect of the fatty acid butyrate produced by bacteria was absent in animals where the HK2 enzyme was removed,” explained Sommer, head of the Functional Host-Microbiome Research working group at the Institute of Clinical Molecular Biology (IKMB) at Kiel University (CAU) and the University Medical Center Schleswig-Holstein (UKSH), Campus Kiel. The results of the study have been published in the renowned scientific journal Cell Metabolism.
Enzyme hexokinase 2: High levels in intestinal cells indicate unhealthy state
In cooperation with scientists from Kiel, Lübeck, Munich and Hanover, as well as from the U.S. and Sweden, Sommer and his team investigated how intestinal bacteria regulate the HK2 enzyme and influence inflammations, including through studies using mice without this enzyme in their intestinal mucosa cells. “These mice were less susceptible to an experimentally-induced intestinal inflammation,” reported the first authors Finn Hinrichsen and Jacob Hamm, both doctoral researchers at the IKMB. The experiments also identified microbial factors that regulate the production of HK2: short-chain fatty acids such as acetate and butyrate. These fatty acids are mainly not absorbed through food, they are produced by bacteria in the intestine—but only by very specific intestinal bacteria. “Acetate increased the concentration of HK2 in the intestinal cells, while butyrate decreased the HK2 levels,” said Sommer. Regarding the inflammation, he noted: “Administering acetate aggravated an experimental intestinal inflammation in wild-type mice, whereas butyrate had a protective effect. These effects were not present in HK2-deficient mice, i.e. animals that lacked the HK2 enzyme.”
Potential target molecule for new anti-inflammatory treatments
The fact that the short-chain fatty acid butyrate, also called butyric acid, stabilizes the intestinal barrier and has an anti-inflammatory effect, was already known from many previous studies. However, the very unpleasant smell of butyrate and its strong laxative effect are obstacles to therapeutic use, for example in cases of chronic inflammatory bowel diseases. “By identifying HK2 as a target of butyrate, we have found a potential entry point for new anti-inflammatory medications. Inhibiting HK2 would be more specific than treatment with butyrate,” emphasized Sommer.
Theoretically, it would also be possible to build up the intestinal microbiome by administering fatty acid-producing bacteria, as pointed out by the last author, Professor Philip Rosenstiel. “To date, many approaches here have been rather broad-based. The study provides an important indication that we may actually be able to specifically select individual bacteria in order to control certain metabolic processes in the intestinal mucosa, and thus also inflammation,” explained IKMB Director Rosenstiel.
Kiel University

