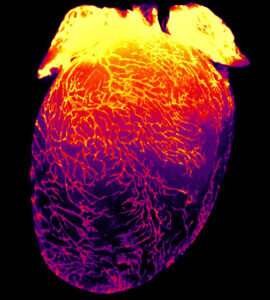
Scientists at the University of North Carolina (UNC) School of Medicine led a study to advance the understanding of lymphatic vessels, which transport lymph, a clear fluid containing important molecules, immune cells and fluid, away from organs and tissues and back to the bloodstream. Although anatomists first described the lymphatic system centuries ago, scientists only recently developed the tools to tease apart its distinct and specialized roles in different organs.
In this study, published in Circulation Research, UNC School of Medicine researchers identified a protein called VE-cadherin as a key factor in the maintenance of lymphatic vessels serving the heart. Deleting VE-cadherin from the lymphatic vessels in newborn and adult lab mice caused the lymphatic vessels in the heart to regress and eventually disappear. They also found that these vessels were not functioning properly. Interestingly, loss of the cardiac lymphatic vessels was not associated with a worsened impairment of heart function, even after an experimentally induced heart attack.
“Everyone had assumed that cardiac lymphatic vessels would be critical for preventing the abnormal buildup of fluid in the heart after injury, but our work suggests that might not be the case,” said study senior author Kathleen Caron, Ph.D., professor and chair of the UNC Department of Cell Biology and Physiology. “Our work also underscores the fact that lymphatic vessels tend to have very specialized functions in different parts of the body.”
The scientists used a line of mice engineered so the gene for VE-cadherin can be selectively deleted from lymphatic cells at a chosen time. Prior studies found that loss of VE-cadherin has profound and diverse effects on the lymphatic vessels found in different organs, such as the gut and skin. However, the specific effects of VE-cadherin loss in lymphatic vessels in the heart had not been described.
In experiments led by UNC-Chapel Hill graduate students Natalie Harris and Natalie Nielsen, the researchers found that deleting the VE-cadherin gene in newborn mice, or even in adult mice, caused severe regression of cardiac lymphatic vessels. This was a striking observation on its own and demonstrated the importance of VE-cadherin in the maintenance of cardiac lymphatics. The researchers also identified some of the key molecular signaling pathways through which VE-cadherin exerts this maintenance effect.
But the researchers also used these cardiac-lymphatic-deficient mice as a new kind of model for studying the role of cardiac lymphatics in normal heart function and heart repair after injury. The findings here were quite unexpected.
Compared to the hearts of mice with normal cardiac lymphatics, the hearts with almost-no-lymphatics appeared to beat and pump blood normally. They did not accumulate fluid and swell up, as often happens to tissues in the body when local lymphatics are disrupted. This was surprising, given that the researchers demonstrated that these VE-cadherin deficient cardiac lymphatics were not functioning normally at baseline. Following an experimentally induced heart attack, the lymphatic-deficient hearts did not function any worse than did hearts with normal lymphatics.
Scientists who study the heart had widely assumed that cardiac lymphatics have important supporting roles in ordinary heart function but also in repair after injury. Numerous prior studies demonstrated that experimentally increasing the growth of cardiac lymphatics after a heart attack improves heart function. It was assumed that this effect was due to a prevention of fluid buildup and swelling, also called edema.
“This interesting mouse model tells us that preventing cardiac edema may not be the primary function of cardiac lymphatic vessels after injury,” said Caron, who is also a member of the UNC McAllister Heart Institute.
Based on their new findings, Caron and colleagues plan to explore whether lymphatic vessels perform other important functions in supporting the heart. For example, the lymphatic system, which includes lymph nodes connected together by a superhighway of lymphatic vessels, helps the immune system detect infections and injuries, mobilize proper responses, and traffic immune cells to and from tissues during the injury and repair process. Caron’s lab is now investigating these potential immune-related functions in heart lymphatic vessels.
University of North Carolina School of Medicine

