Disease models play a major role in COVID-19 research since they allow the study of disease mechanisms or the development of effective and safe vaccines and treatments. In the search for ever better tools, a research group led by Sylvia Knapp at MedUni Vienna has developed a new COVID-19 mouse model, which was recently published in the journal eLife.
Since there are many similarities between the immune systems of mice and humans, mouse models are fundamental research tools of COVID-19 research. However, due to specific genetic and structural differences in the SARS-CoV-2 entry receptor ACE2, mice are not readily infected with virus variants isolated from human COVID-19 patients.
Coronavirus entry point
ACE2 (Angiotensin Converting Enzyme 2) is located in the outer membrane of cells and helps regulating blood-pressure, thus having an important function in the cardiovascular system. In addition, ACE2 is also the gateway for SARS-CoV-2 to enter and infect cells. Whether and how efficiently the virus can infect a species depends on how well the viral spike protein can bind to ACE2. Genetic differences lead to a different ACE2 structure in mice which makes them more resistant to infection with SARS-CoV-2.
Infection possible due to spike mutations
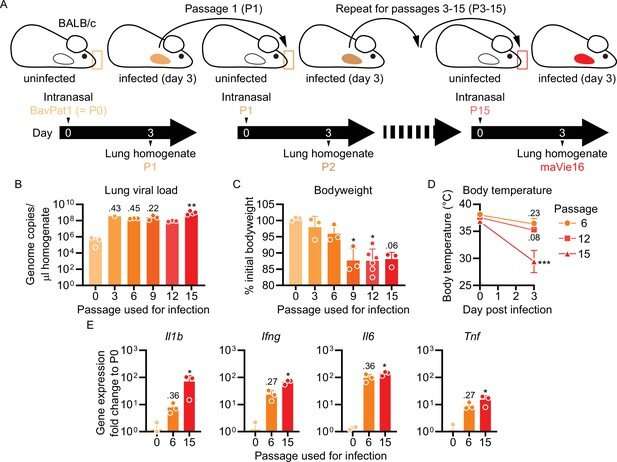
In collaboration with the groups of Josef Penninger (IMBA, Institute of Molecular Biotechnology), Chris Oostenbrink (University of Natural Resources and Applied Life Sciences, BOKU), Andreas Bergthaler and Hannes Stockinger (MedUni Vienna), an interdisciplinary research team led by Sylvia Knapp, head of the Infection Biology Research Laboratory at MedUni Vienna’s Department of Medicine I, has now succeeded in developing a new mouse model for the study of COVID-19. The new model is based on viral mutations of SARS-CoV-2 which result in spike protein alterations that enable the virus to bind murine ACE2. Only three spike protein mutations were necessary to ensure efficient virus infection and replication in mice. The infected animals develop symptoms of COVID-19, allowing the study of disease mechanism and potential treatments.
For instance, the current study demonstrated that synthetic ACE2, administered by inhalation, can protect against SARS-CoV-2 infection. Furthermore, the researchers were able to identify endogenous substances (interferon-g and tumor necrosis factor TNF) that could be inhibited to substantially reduce disease severity.
Advancing COVID-19 research
As the study’s first authors, Riem Gawish and Philipp Starkl from MedUni Vienna’s Department of Medicine I, point out, this new mouse model is a valuable tool for advancing research into the mechanisms of COVID-19, as well as the development of vaccines, drugs and treatments. Unlike existing COVID-19 mouse models, this new tool can be used in common laboratory mouse lines without the need for further manipulation and serves to model both mild and severe disease for research purposes, depending on the viral dosage.
Johannes Angerer, Medical University of Vienna

