Tiny structures called nanoparticles can be used to carry substances to certain parts of the body—for example, to deliver a chemotherapy drug to a tumor. Although such “nanomedicines” offer hope for improving cancer therapeutics, the survival benefits of clinically approved nanomedicines are often modest when compared with conventional chemotherapy. New research published in the Journal of Controlled Release indicates that nanomedicine may provide additional benefits if it’s administered at lower, more frequent doses—called metronomic dosing—rather than the standard maximum tolerated dose of current treatments.
“Nanomedicine and metronomic therapy have been regarded as two different approaches to treat cancer. Our analysis suggests that these two approaches can be viewed using the same unified framework as strategies to enhance treatment,” says co–corresponding author Rakesh K. Jain, Ph.D., director of the E.L. Steele Laboratories for Tumor Biology at Massachusetts General Hospital and the Andrew Werk Cook Professor of Radiation Oncology at Harvard Medical School.
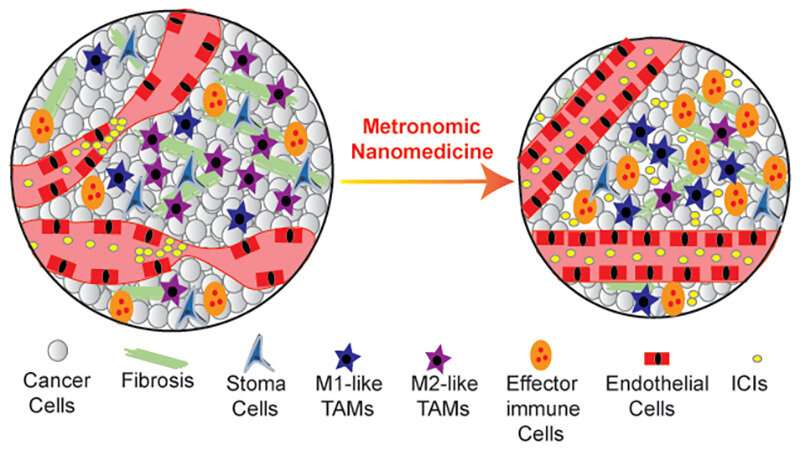
Jain explains that metronomic therapy seems to help normalize the tumor microenvironment—meaning that it helps correct some of the abnormalities that develop around tumors that protect the tumor and foster its growth and spread. For example, while tumors can send out signals that compromise normal blood flow and block immune cell responses (both of which make them hard to treat), metronomic therapy appears to improve blood vessel function and immune activation within a tumor. Recent preclinical studies suggest that nanomedicines can cause similar changes in the tumor microenvironment.
“In this study, we hypothesized that nanoparticle formulations, given the controlled release of their payload and the long blood circulation time, can trigger the same cascade of activities as metronomic therapy,” says Jain.
Using a mathematical framework and experiments conducted in mice, the team showed that both approaches can serve as “normalization strategies” to affect the tumor microenvironment and improve cancer treatments. Also, in mice with triple negative breast cancer or fibrosarcoma, Doxil—a nanomedicine that is approved to treat metastatic breast cancer and consists of doxorubicin encapsulated in a lipid sphere—administered through a metronomic schedule could overcome tumor resistance typically seen when Doxil is given through a standard dosing schedule. A metronomic schedule also improved the efficacy of the combination of Doxil plus a type of immunotherapy called an immune checkpoint inhibitor.
“Nano-immunotherapy, which combines nanomedicines with immunotherapy, has high potential to improve patient outcomes, and for this reason, understanding the mechanisms of resistance to and development of strategies to enhance nano-immunotherapy in breast and other cancer types is urgently needed,” says co–corresponding author Triantafyllos Stylianopoulos, Ph.D., director of the Cancer Biophysics Laboratory and associate professor at the University of Cyprus. “The results of this work could be a basis for the planning of future clinical studies to improve the efficacy of nano-immunotherapy regimens.”
The results suggest that combining nanomedicines with metronomic scheduling can lead to a powerful attack against hard-to-treat tumors. By acting together to normalize the tumor microenvironment, these two strategies give drugs a better chance of reaching cancer cells and targeting them effectively.
The study’s co-authors include Fotios Mpekris and Myrofora Panagi (University of Cyprus), Chrysovalantis Voutouri (Massachusetts General Hospital) and James W. Baish (Bucknell University).
Katie Marquedant, Massachusetts General Hospital

