The loss of muscle mass and strength during aging is called sarcopenia. Affecting the elderly population, sarcopenia is a degenerative process that brings about a decrease in wellbeing and increased dependency. A growing number of studies indicate that this form of muscle atrophy is related to chronic inflammation, but when and why does this inflammation occur?
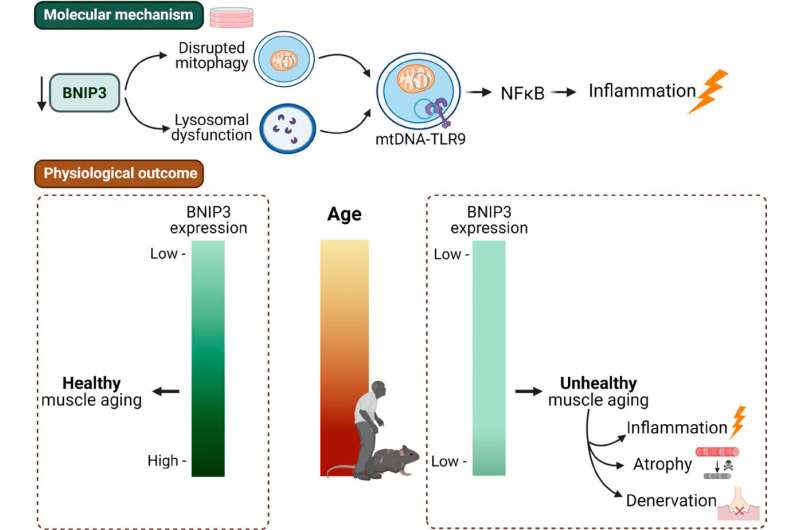
Researchers from IRB Barcelona´s Complex Metabolic Diseases and Mitochondria lab, headed by Dr. Antonio Zorzano, Full Professor of the Faculty of Biology at the University of Barcelona, have discovered that the inflammatory process that causes muscle atrophy is associated with the accumulation of damaged mitochondria in cells. They have also described how the increase in the levels of BNIP3, a protein related to the clearance of damaged mitochondria, is linked to better muscle aging.
“If BNIP3 levels are low at advanced ages, more damaged mitochondria accumulate and this triggers inflammatory processes, which in turn cause muscle atrophy,” explains Dr. David Sebastián, Research Associate in the same laboratory, CIBER researcher, and adjunct professor at the University of Barcelona. “The reason why some people have higher levels of BNIP3 and others lower levels is still unknown,” adds Dr. Andrea Irazoki, first author of the article.
The work has been carried out with cultured cells and mouse models, as well as samples from young and elderly people.
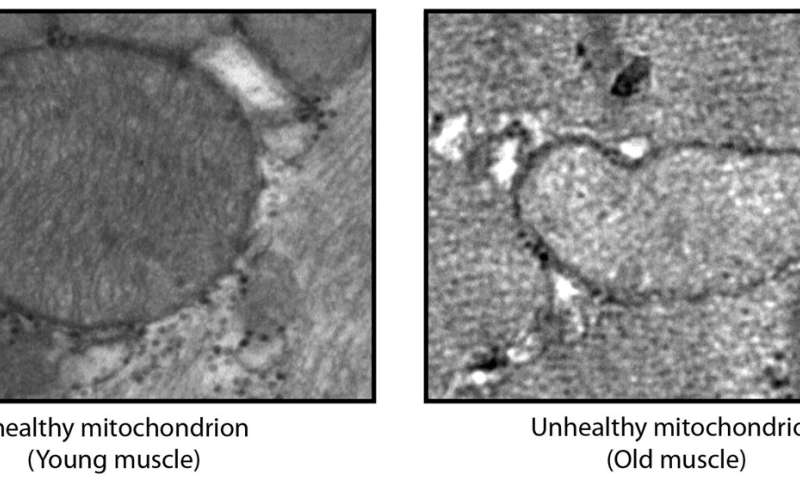
Poor clearance of mitochondria
As the power plants of cells, mitochondria are essential. Cells must therefore ensure that these organelles are in good condition. As mitochondria age and no longer function properly, the body regularly removes or “recycles” them through a process known as mitophagy.
As we age, this process becomes less efficient, and defective mitochondria tend to accumulate. The authors consider that this is compensated by an increase in BNIP3 levels, which stimulate mitophagy. In addition to binding to the mitochondrial membrane to remove these organelles, BNIP3 also affects the function of lysosomes, which are vital for removing the damaged components of cells.
“In this work, we intend to clarify the mechanisms that lead to the development of sarcopenia, and identify tools through which to promote healthy aging at the muscular level,” says Dr. Zorzano, who is also a group leader of the CIBERDEM Programme.
Future laboratory work will focus on the search for blood biomarkers associated with high levels of BNIP3, thus facilitating less invasive analyses, and also on understanding what determines high and low BNIP3 levels (if they have a genetic or environmental origin) and why mitochondria clearance is impaired with age, and the age at which this phenomenon begins.
Institute for Research in Biomedicine (IRB Barcelona)

