Though it has been eclipsed lately by SARS-CoV-2, there is another global epidemic still threatening people: HIV/AIDS. According to UNAIDS, a United Nations initiative, some 38 million people worldwide are currently infected with HIV. Almost as many have died as a result of AIDS since the outbreak of the HIV pandemic in the 1980s. In the search for new approaches to antiviral therapies, scientists at the Helmholtz Institute for RNA-based Infection Research (HIRI) in Würzburg and the Robert Koch Institute (RKI) in Berlin have now developed a new technology that can be used to analyze and impact key stages of the HIV life cycle. Their findings were published today in the journal Nature Structural & Molecular Biology.
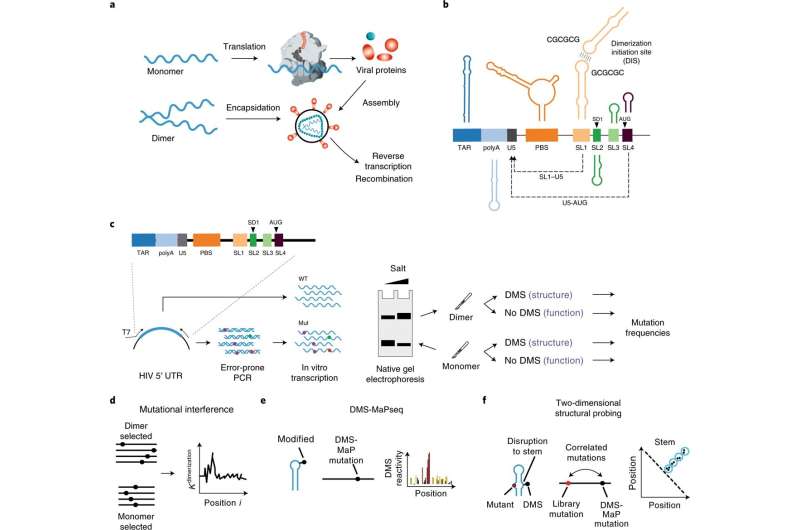
Key stages in the life cycle of a virus can represent attractive targets for drugs and therapies. Therefore, basic research is important to understand and impact the underlying molecular processes. A distinguishing feature of the HIV-1 variant is that it contains two copies of its viral genome. During viral replication two genomes are brought together in a process known as dimerization. The latter is also assumed to be a prerequisite for packaging which will finally lead to new infectious viral particles and complete virus replication.
A molecular switch
In the journal Nature Structural & Molecular Biology, researchers at the Helmholtz Institute for RNA-based Infection Research (HIRI) in Würzburg—an institution of the Helmholtz Centre for Infection Research (HZI) in Braunschweig in cooperation with the Julius-Maximilians-Universität (JMU) of Würzburg—and the Robert Koch Institute (RKI) in Berlin now describe a novel technology to investigate the HIV-1 life cycle at single nucleotide resolution. Baptized FARS-seq (Functional Analysis of RNA Structure), their method helps to identify regions within the HIV-1 genome important for dimerization and virus packaging.
“The idea that dimerization is a prerequisite for packaging has long been discussed in HIV-1 research. However, the underlying molecular mechanisms remained unclear. Our study provides this information in high resolution, allowing targeted intervention,” explains junior professor Redmond Smyth, initiator of the study and research group leader at HIRI.
Liqing Ye conducts research at HIRI in Smyth’s lab and is first author of the current study. She adds, “We were able to show that the genome of HIV-1 exists in two different RNA conformations. Only one of them is involved in genome packaging. In the second conformation, the RNA remains in the host cell to be translated into new viral proteins. These two conformations therefore act like a molecular switch to direct the fate of the viral RNA, and thus viral replication.”
The scientists identified sequences that regulate the equilibrium between these two RNA conformations. Their study illustrates how the binding of viral factors to these regions may be used to target or disrupt viral assembly.
“We hope to be able to leverage these findings into RNA-based antiretroviral drugs or improved gene therapy vectors,” says Redmond Smyth of the Helmholtz Institute in Würzburg. In follow-up studies, he says, the researchers now want to determine whether the observations also apply to other strains of the HI virus.
About HIV
The human immunodeficiency virus (HIV) belongs to the large family of retroviruses. These viruses are protein-coated, and their genome is made of ribonucleic acid (RNA). A characteristic feature of retroviruses, such as HIV, is that each viral particle consists of two copies of the RNA genome. HIV-1 and HIV-2 are the two variants of the virus known to infect humans. The present study addresses HIV-1, which represents more than 90 percent of all infections.
Susanne Thiele, Helmholtz Association of German Research Centres

