Humans have developed four different IgG antibody subclasses that orchestrate protection against invading pathogens. A molecular and cellular understanding of how these contribute to eradication of viral infections may guide design of “super-antibodies” that can become important therapeutic and prophylactic tools against emerging viruses and future pandemics but also for indications beyond infection diseases.
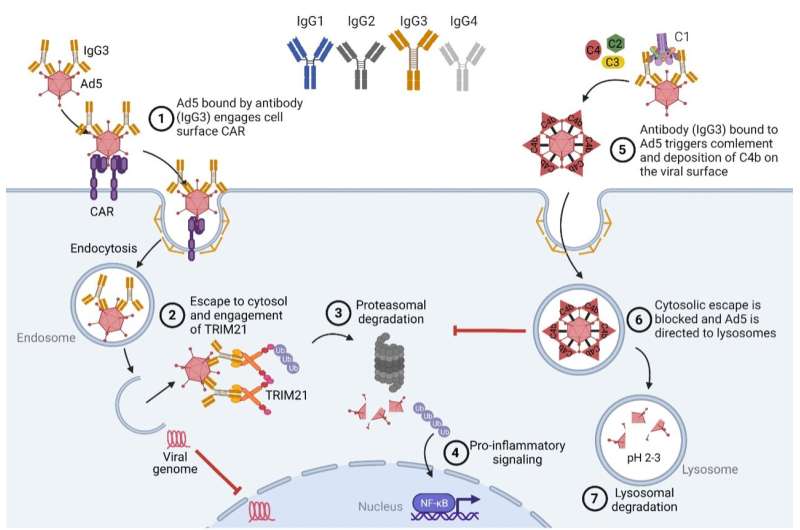
While antibodies are well-known to protect against invading pathogens in the extracellular space, discoveries from the lab of collaborator Leo James at the MRC Laboratory of Molecular Biology have changed this dogma by showing a key role of a cytosolic antibody receptor, TRIM21, in removal of virus before it has had time to replicate and spread. Through a range of studies in collaboration with the Laboratory of Adaptive Immunity, headed by Professor Jan Terje Andersen, at University of Oslo and Oslo University Hospital, new knowledge on the relationship between antibody structure and function has been uncovered.
In the current work, published in Science Immunology, they expand on this by demonstrating that the four human IgG subclasses engage the cytosolic Fc receptor TRIM21 differently followed by targeted degradation of bound virus as well as induction of innate signaling. Importantly, they reveal that IgG3 is superior compared to the three other subclasses, and that this potent activity depends on its elongated and flexible hinge region. In addition, they show that this is also the case for complement-mediated intracellular antiviral protection.
“We only have a minor fraction of IgG3 in our body at any given time, which is related to tight regulation and the fact that it has potent extracellular effector functions. As such, it is a paradox that it is relatively understudied. In this study, we provide a thorough systematic investigation of its unique structural architecture in the context of viral infection. We demonstrate that the long flexible hinge region of IgG3 is required for protective immune functions both in intracellular endosomes as well as in the cytosolic space,” says the study’s first author Stian Foss, Researcher at the Department of Pharmacology, University of Oslo.
“Human IgG3 has been associated with enhanced control or protection against pathogens, including viruses. The study is important as it expands our understanding of IgG3 responses by dissecting its structural composition in the context of antiviral immunity. We demonstrate how this knowledge can be used in engineering of IgG antibodies with altered and even improved activity beyond that of natural IgG3,” says Jan Terje Andersen, the study’s senior author.
University of Oslo

