Last year, scientists at Scripps Research and Toscana Life Sciences studied the blood of 14 COVID-19 survivors to find the most potent antibodies against the SARS-CoV-2 virus. One of the leading molecules that emerged—now in stage II/III trials in Italy—was an antibody dubbed J08, which seemed to be capable of both preventing and treating COVID-19.
Now, the same group—a collaboration between scientists at Scripps Research and in Italy and France—has visualized exactly how J08 binds to different SARS-CoV-2 variants in different conformations, explaining what makes the monoclonal antibody so potent. The research, published in Proceedings of the National Academy of Sciences, suggests that the J08 antibody, because of its flexibility, will likely remain effective against future variants of COVID-19.
“Even though we can’t predict what variants of COVID-19 will emerge next, understanding the details of J08 reveals what works against the virus, and perhaps how we can engineer antibodies to be even more potent,” says senior author Andrew Ward, Ph.D., professor of Integrative Structural and Computational Biology at Scripps Research.
When a person is exposed to a virus like SARS-CoV-2, their body generates a variety of antibodies that bind to different sections of the virus to clear it from the body. Scientists designing vaccines and treatments against COVID-19 are interested in what makes some of these naturally produced antibodies—like J08—more effective than others. In the months after Ward and his collaborators first identified J08, it became clear that the antibody, unlike many others, was potent against a variety of COVID-19 variants.
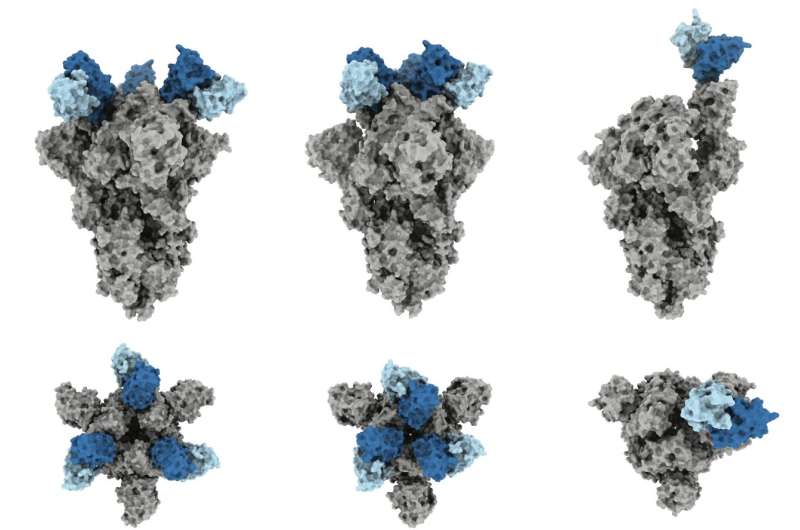
In the new work, the researchers determined the three-dimensional structure of J08 as it bound to the spike protein of SARS-CoV-2. They confirmed that J08 successfully attached to the Alpha, Beta, Gamma and Delta variants and neutralized the viruses—preventing them from replicating. However, J08 attached to the Omicron variant about 7 times more slowly, and then rapidly came off. About 4,000 times more J08 was needed to fully neutralize Omicron SARS-CoV-2 compared to the other variants.
“With variants other than Omicron, this antibody binds quickly and doesn’t come off for hours and hours,” says co-first author Gabriel Ozorowski, a senior staff scientist in the Ward lab at Scripps Research. “With Omicron, we were initially happy to find that it still binds, but it falls off very quickly. We identified the two structural changes that cause this.”
The team showed that, for all the variants, J08 binds to a very small section of the virus—a section that generally stays the same even as the virus mutates. Moreover, J08 could attach in two completely different orientations, like a key that manages to unlock a door whether it is right side up or upside down.
“This small, flexible footprint is part of why J08 is able to withstand so many mutations—they don’t impact the antibody binding unless they happen to be in this one very small part of the virus,” says co-first author Jonathan Torres, lab manager of the Ward lab at Scripps Research.
The Omicron variant of SARS-CoV-2, however, had two mutations (known as E484A and Q493H) that changed the small area of the virus that directly interfaces with J08, anchoring it in place. Ward and his collaborators found that if just one of these mutations is present, J08 still manages to bind and neutralize the virus strongly, but mutations in both are what make it less effective against the Omicron variant.
The researchers say the new results support the continued clinical trials of the monoclonal antibody based on J08.
“I think we’re pretty confident that future variants won’t necessarily have both of these two critical mutations at the same time like Omicron,” says Ozorowski, “so that makes us hopeful that J08 will continue being very effective.”
The Scripps Research Institute

