The liver is an essential organ that takes care of clearing toxins in our bodies. Because it constantly deals with toxic substances, it is likely to be regularly injured. To overcome this, the liver has a unique capacity among organs to regenerate itself after damage. Because a lot of the body’s ability to heal itself and regenerate decreases as we age, scientists were wondering if the liver’s capacity to renew also diminishes with age.
In terms of past research, the nature of liver renewal in humans remained a bit of a mystery while animal models provided contradictory answers. “Some studies pointed to the possibility that liver cells are long-lived while others showed a constant turnover. It was clear to us that if we want to know what happens in humans, we need to find a way to directly assess the age of human liver cells,” says Dr. Olaf Bergmann, research group leader at the Center for Regenerative Therapies Dresden (CRTD) at TU Dresden.
The human liver remains a young organ
The interdisciplinary team of biologists, physicists, mathematicians, and clinicians led by Dr. Bergmann analyzed the livers of multiple individuals who died at ages between 20 and 84 years old. Surprisingly, the team showed that the liver cells of all subjects were more or less the same age.
“No matter if you are 20 or 84, your liver stays on average just under three years old,” explains Dr. Bergmann. The results show that the adjustment of liver mass to the needs of the body is tightly regulated through the constant replacement of liver cells and that this process is maintained even in older people. This ongoing liver cell replacement is important for various aspects of liver regeneration and cancer formation.
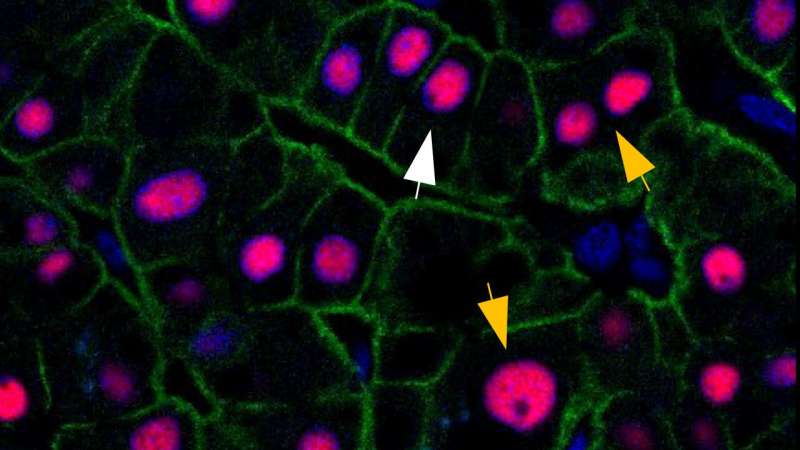
Liver cells with more DNA renew less
However, not all the cells in our liver are that young. A fraction of cells can live up to 10 years before renewing itself. This subpopulation of liver cells carries more DNA than the typical cells. “Most of our cells have two sets of chromosomes, but some cells accumulate more DNA as they age. In the end, such cells can carry four, eight, or even more sets of chromosomes,” explains Dr. Bergmann.
“When we compared typical liver cells with the cells richer in DNA, we found fundamental differences in their renewal. Typical cells renew approximately once a year, while the cells richer in DNA can reside in the liver for up to a decade,” says Dr. Bergmann. “As this fraction gradually increases with age, this could be a protective mechanism that safeguards us from accumulating harmful mutations. We need to find out if there are similar mechanisms in chronic liver disease, which in some cases can turn into cancer.”
Lessons from the nuclear fallout
Determining the biological age of human cells is a massive technical challenge, as methods commonly used in animal models cannot be applied to humans.
Dr. Bergmann’s group specializes in retrospective radiocarbon birth dating and uses the technique to assess the biological age of human tissues. Carbon is a chemical element that is ubiquitous and forms the backbone of life on Earth. Radiocarbon is one of a variety of types of carbon. It appears naturally in the atmosphere. Plants incorporate it through photosynthesis, in the same way as typical carbon, and pass it on to animals and humans. Radiocarbon is weakly radioactive and unstable. These characteristics are taken advantage of in archeology to determine the age of ancient samples.
“Archeologists have used the decay of radiocarbon successfully for many years to assess the age of specimens, one example being dating of the shroud of Turin,” says Dr. Bergmann. “The radioactive decay of radiocarbon is very slow. It provides enough resolution for archeologists but it is not useful for determining the age of human cells. Nevertheless, we can still take advantage of the radiocarbon in our research.”
The aboveground nuclear tests carried out in the 1950s introduced massive amounts of radiocarbon into the atmosphere, into the plants, and into the animals. As a result, cells formed in this period have higher amounts of radiocarbon in their DNA.
Following the official ban of aboveground nuclear testing in 1963, the amounts of atmospheric radiocarbon started to drop and so did the amounts of radiocarbon incorporated into the animal DNA. The values of atmospheric and cellular radiocarbon correspond to each other very well.
“Even though these are negligible amounts that are not harmful, we can detect and measure them in tissue samples. By comparing the values to the levels of atmospheric radiocarbon, we can retrospectively establish the age of the cells,” explains Dr. Bergmann.
Unparalleled insights directly from the source
The Bergmann group also explores the mechanisms that drive the regeneration of other tissues considered as static, such as the brain or the heart. The team has previously used their expertise in retrospective radiocarbon birth dating to show that the formation of new brain and heart cells is not limited to prenatal time but continues throughout life. Currently, the group is investigating whether new human heart muscle cells can still be generated in people with chronic heart disease.
“Our research shows that studying cell renewal directly in humans is technically very challenging but it can provide unparalleled insights into the underlying cellular and molecular mechanisms of human organ regeneration,” concludes Dr. Bergmann.
The research was published in Cell Systems.
Dresden University of Technology

