Researchers from Skoltech and Karfidov Lab—a startup co-founded by an Institute alumnus—have revealed flaws in the product development process traditionally used in the medical technology industry. Based on a case study of a drug injector, the team reports challenges exacerbated by the pandemic and makes scientifically grounded proposals for improvement of medtech product development. The research was published in the Proceedings of the Design Society following presentation at the International DESIGN 2022 Conference.
The COVID-19 pandemic has exposed preexisting flaws in the conventional process of medical device development. A recent Skoltech study formally describes this traditional process using the example of a drug injection device produced by Karfidov Lab. It highlights typical shortcomings and suggests solutions inspired by analyzing medtech companies and based on engineering design science, which is an actively growing discipline in such places as MIT, Stanford, the Technical University of Munich, the Technical University of Denmark, and the University of Cambridge.
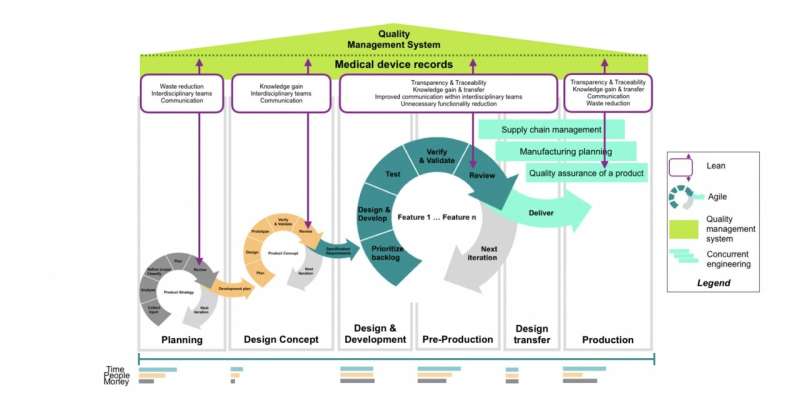
“For the case study, we conducted detailed design interviews with team members in the company, seeking to understand how they approached product development and how they collaborated within their team, how everyone understood their tasks and what the results of their work were,” Skoltech Ph.D. graduate and Research Scientist Yaroslav Menshenin, who co-authored the study, explained. “It soon became clear there were things that could be improved.” The Skoltech team proposed ways to improve the efficiency of the process with a Lean-Agile methodology incorporating elements of concurrent engineering. The approach was inspired by the industrial and research best practices, cast in a more systematic light.

The researchers believe that by introducing a framework similar to their Lean-Agile approach before product development begins, a company can eliminate inconsistencies and efficiently transfer knowledge accumulated over the course of product development to any new team members getting on board. “Everyone has to be on the same page both before development begins and at any other point in the product life cycle,” Skoltech graduate and co-founder of Karfidov Lab Dmitry Vasilev added.
Approvals by the Food and Drug Administration and other regulatory bodies proved to be another significant bottleneck. “Sure, COVID turned out to be a demonstration of supply chain vulnerability, but also of how a concerted effort to develop even such a complex product as a vaccine and bring it to market could really speed things up,” Skoltech Ph.D. candidate and lead author of the paper Natalia Glazkova said. “Both the industry and the customers want things to happen faster, and there are signs the regulatory authorities may be willing to embrace this. COVID has shown it to be possible.”
Another common problem, according to the researchers, is “trying to implement everything at once.” This can be mitigated with Agile loops. “Ideally, you should start out with just the critical functionality—say, drug injection without medical assistance—and then gradually add on new features as you go,” Glazkova explained. “You would also have early quality management in place and make ‘semifinal’ decisions at every stage, each matching a new feature.”
Skoltech Professor Clement Fortin, the principal investigator of the study, summed up the main points: “In the Systems Thinking group, we’re looking for flexible ways to develop more competitive products that would be ready sooner for the market. To achieve this, we’re proposing to focus on improving the development processes and software tools. Our solution is scientifically grounded and inspired by a broad analysis of best research practices and a specific case study.”
Skolkovo Institute of Science and Technology

