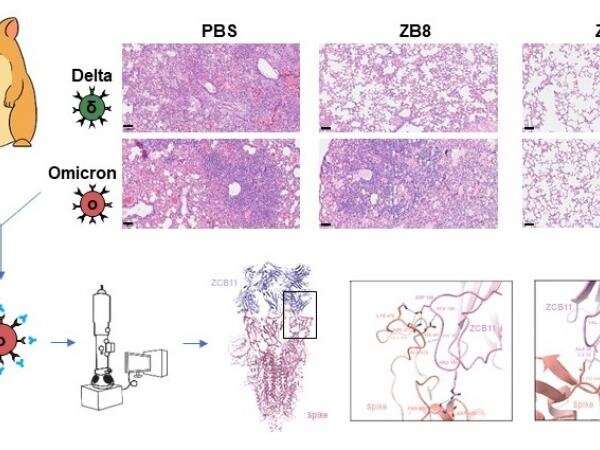
Structural biologists at The Hong Kong University of Science and Technology (HKUST) together with researchers at the AIDS Institute, The University of Hong Kong (HKU), Department of Microbiology, School of Clinical Medicine, the LKS Faculty of Medicine of The University of Hong Kong (HKUMed) and the State Key Laboratory of Emerging Infectious Diseases, HKU have demonstrated that ZCB11, a broadly neutralizing antibody derived from a local mRNA-vaccinee against the spreading omicron variants of SARS-CoV-2, displays potent antiviral activities against all variants of concern (VOCs), including the dominantly spreading omicron BA.1, BA1.1 and BA.2. Critically, either prophylactic or therapeutic ZCB11 administration protects lung infection against omicron viral challenge in golden Syrian hamsters. The research paper is now published online in Nature Communications.
The strikingly high transmissibility and antibody evasion of SARS-CoV-2 omicron variants have posed great challenges to the efficacy of current vaccines and antibody immunotherapy. In response to the continuous emergence of SARS-CoV-2 omicron variants with unpredictable pathogenicity, universal masking, quarantine and endless viral testing have to be maintained, resulting in social anxiety and economic disruption. It is therefore important to investigate whether host immune response can generate broadly neutralizing antibodies, which is essential not only for antibody-based immunotherapy but also for vaccine optimization to induce equally broad protection.
Research methods and findings
In this study, the HKUMed team has established an effective platform of cloning technology that natively pairs antibody genes from individual human memory B cells. Using this technique, the research team successfully discovered ZCB11 after screening 34 BNT162b2-vaccinees in Hong Kong, and demonstrated that ZCB11 neutralizes all VOCs including alpha (B.1.1.7), beta (B.1.351), gamma (P1), delta (B.1.617.2) and omicron (B.1.1.529) by testing both pseudoviruses and authentic live viruses. Importantly, ZCB11 administration protects lung infection against both live omicron and delta viral challenges in golden Syrian hamsters respectively, under both prophylactic and therapeutic conditions. Furthermore, the HKUST collaborative team deciphered the complex structure of ZCB11 and spike protein at atomic resolution using single particle cryo-EM, revealing the unique molecular mode of ZCB11 action, which lays a solid foundation for upcoming structure-guided antibody and vaccine optimization.
“The findings suggested that ZCB11 is a promising antibody drug for biomedical interventions against pandemic SARS-CoV-2 variants of concern,” remarked Professor Chen Zhiwei, Director of AIDS Institute and Professor of the Department of Microbiology, School of Clinical Medicine, HKUMed, who led the study. “Although our findings implicate that the HKUMed team is at the world’s forefront of research and development of human antibody drugs and vaccines against COVID-19, we still urgently need to establish large-scale manufacturing capacity and clinical translational hubs in Hong Kong, in order to meet its aspiration of becoming an international innovation center.”
“The high-resolution structural information enabled us to understand the molecular mechanism of ZCB11 responding to a broad SARS-CoV-2 variant of concern,” said Professor Dang Shangyu, Assistant Professor of Division of Life Science, HKUST. “This study relies on the state-of-the-art cryo-EM facility at HKUST, which demonstrated its capability to support not only research in structural biology, but also many other research fields, such as antibody development in this study.”
Hong Kong University of Science and Technology

