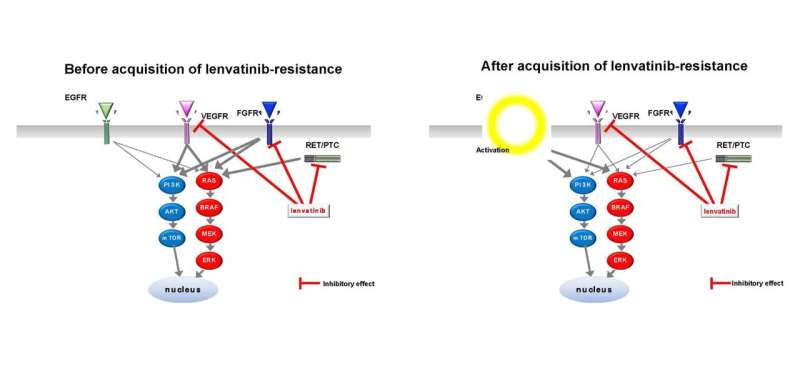
The multitargeted tyrosine kinase inhibitor (MKI), lenvatinib, is widely used to treat advanced and metastatic thyroid cancer. However, the molecular mechanisms by which thyroid cancer cells become resistant to lenvatinib remain poorly understood. In this study, we demonstrated, for the first time, that activation of the epidermal growth factor receptor (EGFR)-mediated signaling pathway is involved in lenvatinib resistance in thyroid cancer cells.
Although thyroid cancer is the most common endocrine malignancy, few effective drugs are currently available. Lenvatinib, an MKI, has been widely used for the treatment of advanced and metastatic thyroid cancer. Unfortunately, some cancers are resistant to lenvatinib from the beginning of the treatment or become resistant to the treatment. The mechanisms underlying lenvatinib resistance must therefore be elucidated to develop better and more effective strategies for treatment of advanced thyroid cancer. Using cultured thyroid cancer cell lines, researchers from the Division of Breast and Endocrine Surgery, Department of Surgery, Shinshu University School of Medicine, Japan, studied these mechanisms at the molecular level.
The researchers established lenvatinib-resistant sublines (namely, TPC-1/LR and FRO/LR) from two parental thyroid cancer cell lines (TPC-1 and FRO) and found that phosphorylation of EGFR, extracellular signal-regulated kinase (ERK), and Akt was promoted in TPC-1/LR cells. Furthermore, activation of the EGFR pathway by administrating epidermal growth factor (EGF) to the parental lines TPC-1 and FRO before they acquired resistance induced a decrease in lenvatinib sensitivity. In contrast, administration of the EGFR inhibitor, lapatinib, simultaneously with lenvatinib to TPC-1/LR cells, in which EGFR phosphorylation is upregulated, enhanced the growth inhibitory effect of lenvatinib.
Next, in nude mouse xenograft models transplanted with lenvatinib-resistant TPC-1/LR cells, treatment with lenvatinib in combination with lapatinib inhibited tumor growth more remarkably than lenvatinib monotherapy did.
Furthermore, when the effects of lenvatinib on signal transduction pathways were analyzed in six thyroid cancer cell lines, enhancement of EGFR phosphorylation was observed in all the cell lines, regardless of the histological type of origin of the cell lines or presence of driver gene mutations. However, the level of phosphorylation of downstream signaling molecules, such as ERK and Akt, differed among cell lines.
To verify whether EGFR inhibition by lapatinib therapy in combination with lenvatinib enhanced the growth inhibitory effect of lenvatinib, both drugs were simultaneously administered to these six cell lines. Consequently, synergistic enhancement of growth inhibition was observed in three cell lines that were initially less sensitive to lenvatinib.
Lenvatinib exerts its antitumor effects mainly by potently inhibiting activity of vascular endothelial growth factor receptor 1–3 (VEGFR1–3), fibroblast growth factor receptor 1–4 (FGFR1–4), and rearranged during transfection (RET) genes. The results of this study suggest that EGFR, a tyrosine kinase receptor (TKR), which is not a target molecule of lenvatinib, is activated during lenvatinib administration and may confer lenvatinib resistance in thyroid cancer cells. This study demonstrated that inhibition of the EGFR pathway by a combination of lenvatinib and EGFR inhibitor might be a new therapeutic strategy to increase lenvatinib sensitivity and overcome lenvatinib resistance in thyroid cancer.
Thyroid cancer is classified into the following types: differentiated thyroid cancer and anaplastic thyroid cancer arising from follicular epithelial cells and medullary carcinoma arising from parafollicular cells. Although anaplastic thyroid cancer accounts for only approximately 2% of the thyroid cancers, its prognosis is extremely poor. In contrast, approximately 90% of thyroid cancers are differentiated thyroid cancers, including papillary and follicular carcinomas. The standard treatment for differentiated thyroid cancer is surgery and radioactive iodine therapy, with a favorable 10-year disease-specific survival rate of > 90%. However, some cancers are resistant to radioactive iodine therapy, and the 10-year survival rate for differentiated cancers that have metastasized to distant organs is < 50%.
Driver gene mutations, such as BRAF, RAS, and RET/PTC mutations, are frequently found in thyroid cancer cells. However, activation of mitogen-activated protein kinase signaling pathways downstream of TKRs, such as FGFR, VEGFR, or platelet-derived growth factor receptor (PDGFR), is observed in thyroid cancers, regardless of the presence or absence of driver gene mutations. Moreover, MKIs have been used to treat differentiated thyroid cancers that have become refractory to radioactive iodine therapy. Lenvatinib, an oral MKI that targets VEGFR1-3, FGFR1-4, PDGFR, and RET, has been widely used worldwide because it extended progression-free survival in a phase III study of radioactive iodine-refractory differentiated thyroid cancer.
However, major clinical challenges, such as some cancers being resistant to lenvatinib from the beginning of the treatment and the emergence of resistance during treatment, continue to prevail. Therefore, to overcome lenvatinib resistance, identifying biomarkers that predict lenvatinib sensitivity and elucidating the molecular mechanisms by which thyroid cancer cells acquire resistance to lenvatinib are necessary.
- Establishment of lenvatinib-resistant cell lines: The thyroid cancer cell lines TPC-1 (with RET/PTC mutation) and FRO (with BRAFV600E mutation) were cultured in the presence of lenvatinib for an extended period; the resistant lines TPC-1/LR (140-fold resistant compared to parental cells) and FRO/LR (5.3-fold resistant compared to parental cells) were established and used in the study.
- Determination of changes in intracellular signaling pathways: Protein expression and phosphorylation of signaling molecules were analyzed using western blotting (WB) to determine the mechanism by which the acquisition of lenvatinib resistance alters intracellular signal transduction.
- Drug sensitivity analysis: The growth inhibitory effect of lenvatinib alone, in the presence of EGF, and in combination with lapatinib, on thyroid cancer cell lines was analyzed using the water-soluble tetrazolium salt (WST-1) assay.
- In vivo analysis of antitumor effects of drugs: Lenvatinib-resistant TPC-1/LR cells were implanted subcutaneously into nude mice to establish a tumor-bearing model, and the antitumor effects of lenvatinib alone and in combination with lapatinib were analyzed. In addition, tumor tissues were collected and subjected to WB and immunohistochemistry.
Propagation effect and future plans
Lenvatinib has been widely used for the treatment of advanced and metastatic differentiated thyroid cancers resistant to radioactive iodine therapy. However, no effective treatment strategy has been established once the cancer becomes resistant to this drug. The results of this study demonstrated that a combination of lenvatinib and an EGFR inhibitor enhances the efficacy of lenvatinib and can be a new treatment strategy for advanced thyroid cancer.
The combination of a BRAF inhibitor and an MEK inhibitor has been used in clinical practice overseas for BRAF V600E mutation-positive anaplastic thyroid cancer, and the therapeutic strategy of inhibiting signaling pathways critical for growth by combining multiple molecular targeting agents has been used in clinical practice for several other malignancies. The results of this study strongly suggest that the combination of multiple molecular-targeted agents will play an important role in future strategies for treating malignant tumors.
The team plans to explore other lenvatinib-resistant mechanisms in parallel with the molecular mechanisms by which lenvatinib induces EGFR activation in thyroid cancer cells.
The research was published in Cancer Science.
Shinshu University

