Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes multi-organ damage, including liver dysfunction identified in more than 50% of COVID-19 patients. Liver damage in COVID-19 patients could be attributed to cytopathic effects induced by the interaction between the virus and liver cells, to an increased immune response or to drug toxicity associated with the treatment of these patients, according to research carried out at CIC bioGUNE.
Since the beginning of the pandemic, the impact of SARS-CoV-2 on the liver has been widely debated. “Clinical studies conducted in patients with COVID-19 described liver lesions, however, it was not clear whether the observed damage was a direct consequence of SARS-CoV-2 tropism to this organ or if, on the contrary, it was the result of the administration of the antibiotic/antiviral treatments used in these patients,” says Maria Mercado-Gómez, first author of the publication. In this context, “other viruses targeting the upper respiratory tract, such as SARS-CoV and MERS-CoV, have shown tropism towards the liver, so we wonder if the new coronavirus could do so as well,” explains Dr. Prieto-Fernández.
The groups of Dr. Martínez-Chantar and Dr. Asís Palazón at CIC bioGUNE, synergized their expertise in hepatic and immunological research to demonstrate that hepatocytes are susceptible to infection in different models, i.e., primary hepatocytes derived from humanized angiotensin-converting enzyme-2 (hACE2) mice and primary human hepatocytes. This study is also the result of collaboration with the laboratories of Dr. Jiménez-Barbero, Dr. Nogueiras, Dr. Vicent Prevot and Dr. Félix Elortza, and results have been published in Communications Biology, on August 17, 2022.
“We used pseudotyped viral particles decorated with SARS-CoV-2 spike particles that induced the expression of ZsGreen in host cells upon infection, a fluorescent protein that allows quantification of infection by flow cytometry,” explains Dr. Prieto-Fernandez.
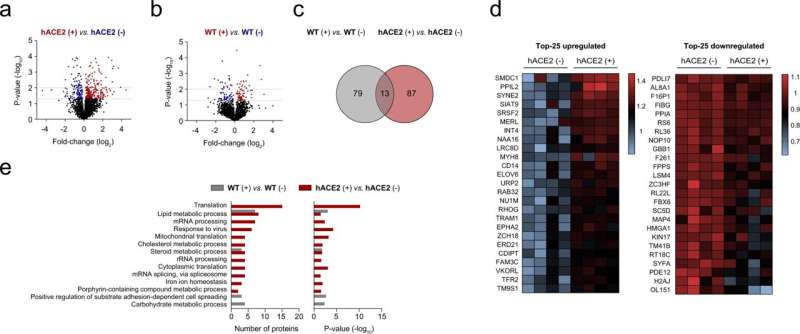
After demonstrating the interaction between the spike of SARS-CoV-2 and hepatocytes, the researchers studied the metabolic impact of this interaction using a battery of experimental procedures, including metabolic flux experiments employing carbon-labeled glucose and proteomic analysis by liquid chromatography-tandem mass spectrometry. This multi-method approach led to the discovery that the SARS-CoV-2 spike promotes metabolic reprogramming in hepatocytes toward glycolysis, but also impaired mitochondrial activity, which partly explains the liver damage associated with COVID-19.
“Importantly, we found that primary human and hACE2 hepatocytes, in which steatosis and inflammation were induced by methionine and choline deprivation, are more vulnerable to infection, and these data support the predisposition of patients with metabolically associated fatty liver disease, MAFLD, to a more severe prognosis of COVID-19,” said Dr. Malu Martinez-Chantar. In this context, metformin, a common therapeutic option for hyperglycemia in patients with type 2 diabetes that is known to partially attenuate fatty liver, reduces human and mouse hepatocyte infection.
In summary, the research provides evidence that hepatocytes are susceptible to SARS-CoV-2 pseudovirus infection and propose that metformin could be a therapeutic option to attenuate SARS-CoV-2 infection in patients with fatty liver.
CIC bioGUNE

