Researchers at the Francis Crick Institute have developed a method that could help predict whether people with lung cancer will respond to chemotherapy, based on which genes are switched on in their tumor.
In recent years, cancer researchers have increased our understanding of how particular gene mutations impact how a tumor develops, evolves and responds to treatment. However, the presence of mutations alone does not provide a complete picture of the tumor’s biology.
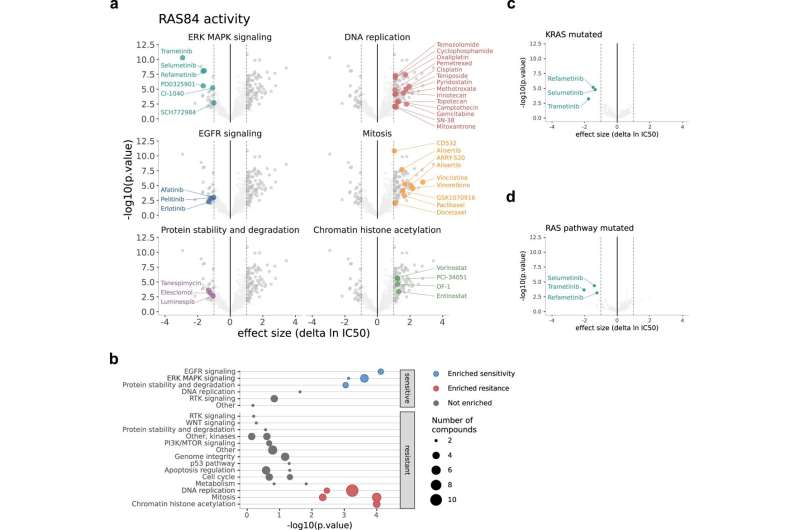
In their study, published in Nature Communications this week, the scientists looked at expression of different genes within tumors, which ones are switched on or off.
Focusing on genes associated with the RAS signaling pathway, the researchers analyzed datasets containing information on the activity of these genes in cancer cells. From these datasets and with the help of AI, they compiled a unique gene signature of 84 genes.
When the scientists looked retrospectively at data from over 500 lung cancer patients who had already been through treatment, the gene signature was able to correctly predict which patients would have and would not have benefitted from chemotherapy.
Sophie de Carné, co-lead author and postdoctoral research scientist in the Oncogene Biology laboratory at the Crick, says, “With many cancer therapies on offer, and more drugs on trial, it is a challenge to predict which treatment has the highest chance of success for a particular patient. Because we know that RAS signaling is involved in various drug-resistance mechanisms, we hope our gene signature will help define groups of patients who could benefit from specific therapies.
“In the meantime, there is lots of work that needs to be done to reach this goal. It needs to be tested in a trial setting, and we are also adapting it to hopefully make it easier to be deployed.”
Julian Downward, co-lead author and principal group leader of the Oncogene Biology laboratory at the Crick, says, “Our work shows that the RAS signaling pathway is a crucial factor in explaining why, in lung cancer patients, there is varied response to chemotherapy. Levels of gene expression in this pathway is more decisive than the presence of certain gene mutations, including a mutation in the KRAS gene.”
Philip East, first author and deputy head of the bioinformatics and biostatistics platform at the Crick, adds, “Thanks to advancing computational tools we can tap into the immense amount of existing data on cancer. This study shows how these datasets can lead to new insights that have potential to improve outcomes for patients.”
The researchers will continue this work, including studying whether their gene signature could also be used to predict which patients are most likely to benefit from immunotherapy. And while this gene signature was optimized for lung cancers, the method could be used to develop signatures for other cancers.
The Francis Crick Institute

