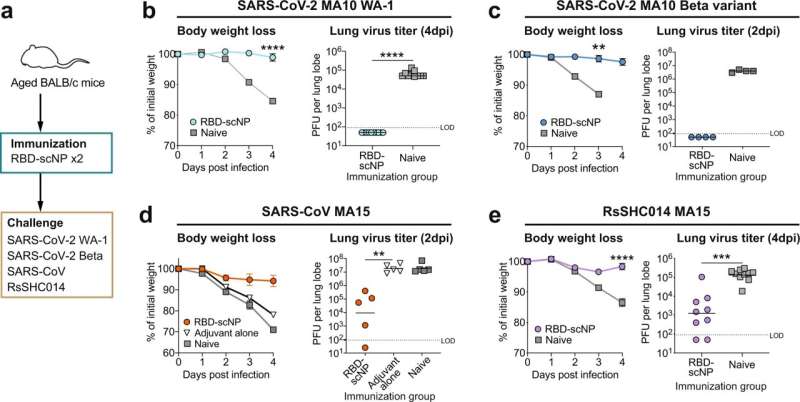
In laboratory and primate tests, a new pan-coronavirus vaccine developed by the Duke Human Vaccine Institute (DHVI) has demonstrated wide protection against SARS-CoV-2 viruses and variants, including omicron BA.5.
Publishing in the journal Nature Communications, the DHVI and collaborating researchers reported that three doses of the pan-coronavirus vaccine induced neutralizing antibodies against beta, delta and omicron variants, including the subvariant BA.5.
The vaccine was also tested by exposing vaccinated animals to various SARS-CoV-2 strains. It demonstrated protection against infection from the original SARS-CoV-2 strain, as well as beta and delta variants.
“This provides proof-of-concept for a first generation pan-SARS-like virus vaccine,” said Barton F. Haynes, M.D., director of the DHVI.
The vaccine candidate is a combination of a nanoparticle antigen developed at Duke, along with an adjuvant—an ingredient that boosts a vaccine’s effects—formulated by the Access to Advanced Health Institute. The adjuvant formulation, 3M-052-AF, significantly enhanced the immune responses in the animals when combined with the antigen.
“While SARS-CoV-2 continues to mutate during the ongoing pandemic, there are conserved regions on the virus that our vaccine will continue to successfully bind to, regardless of mutations,” Haynes said. “That will be critical for present and future protection.”
The vaccine candidate will now move to production and an initial Phase I clinical trial in humans. Ongoing research will focus on developing the vaccine as a booster for the currently vaccinated population, aiming to optimize the induction of antibodies that would neutralize new variants.
Sarah Avery, Duke University

