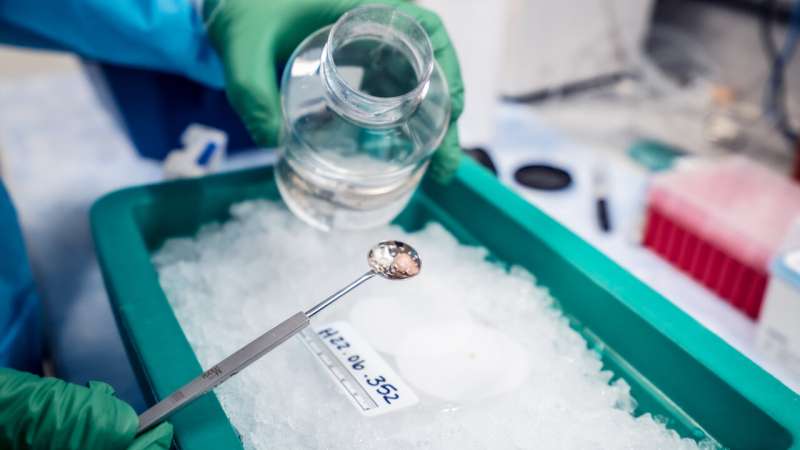
If an epilepsy patient needs brain surgery, their brain surgeon often extracts a piece of tissue the size of a sugar cube from the outermost layer to access the regions responsible for the seizures. This excised lump is typically discarded as medical waste since it is far from the diseased site. But to neuroscientists like Jonathan Ting, Ph.D., this brain nugget is “the most precious piece of matter in the universe.”
Ting, Associate Investigator at the Allen Institute for Brain Science, a division of the Allen Institute, and his team receive brain tissue removed during surgery and willingly donated by patients to uncover the workings of living human brain cells. Ting and others at the Allen Institute for Brain Science aim to build a “periodic table” of brain cell types to categorize the brain by its cellular building blocks. Understanding what happens at the cellular level can help scientists better understand the larger experiences in the mind, including learning, consciousness, and even psychedelic experiences.
For the past two years, Ting and his colleagues have been sending brain samples on trips with magic mushrooms.
By dosing the excised pieces of brain with psilocybin, the psychoactive ingredient in hallucinogenic shrooms, the team wants to understand how individual neurons respond to the drug. There is growing evidence and ongoing trials that show psilocybin as a potential therapeutic for depression, anxiety, PTSD and other psychiatric conditions, Ting said. However, little is known about how psilocybin works in the human brain, either its hallucinatory actions or its ability to ameliorate psychiatric disorders.
“It’s striking that all of this work is ongoing in the clinic on human patients without a deep understanding of what the drug does at the mechanistic level,” Ting said. “Our idea is to study them at the single-cell level and try to see what these drugs are doing in specific brain cell types and regions.”
Psilocybin mimics serotonin, a neurochemical messenger that cells release to regulate mood, and binds to certain kinds of serotonin receptors on various brain cells, said Meanhwan Kim, Ph.D., Ting’s colleague and a neuroscientist at the Allen Institute for Brain Science. To look at what happens to cells exposed to the drug, the team used a technique called Patch-seq to capture electrical activity, 3D shape and gene expression of individual neurons bathed in psilocybin.
They hypothesized that the psychedelic drug would hyperactivate all the cells carrying the specific serotonin receptor, but instead, some of these cells activated, some deactivated and, notably, most did not respond. These receptors are present in multiple parts of the brain so the scientists are now broadening their search to sample cells from different regions, as well as studying the same neurons in mice, where they’re also developing new tools to home in on these specific cell types.
The team is presenting their findings Saturday Nov. 12 at the Society for Neuroscience 2022 conference in San Diego. Although they don’t have an explanation for these findings yet, bringing awareness to the peculiar cellular mechanisms of psilocybin might lead to further research on how the drug works and what it can be used for.
Can we separate the trip from the medicine?
Psilocybin is a Schedule I substance, deemed by the United States Drug Enforcement Administration as highly addictive and difficult to obtain for medical use and research purposes under the Controlled Substances Act. It took the researchers almost a year to gain the licensing to use the drug, and the team is required to keep it in a passcode-protected safe in the lab.
But changing perceptions about psilocybin and other psychedelics are driving “a renaissance in psychedelic research,” said Christof Koch, Ph.D., chief scientist of the Allen Institute’s MindScope Program, who is also part of the team studying the drug’s action.
With the help of psychotherapists, patients using the drug report a dissolution of their sense of self and feel connected with the universe, gaining a positive outlook on life, said Koch. Such actions could underlie psychedelics’ ability to treat conditions like depression and anxiety.
“Having these mystical experiences, the patient is able to overcome their depression or reframe that depression and return to a more baseline mental being,” Koch said. “It really seems to restore sort of the wellness and balance in the life of the patient. It’s quite magical”
Ting wonders if scientists can separate the trip from the medicine. If so, would the stigma against psychedelics resolve? But to Koch, the two features may be inseparable.
“We don’t know yet, but I strongly suspect that you cannot separate the two. Hallucinating is an essential part of the way these drugs work,” Koch said.
It’s not clear yet how long these therapeutic effects last. Many studies only looked up to six months after treatment, Koch said, adding that more research is needed to measure psilocybin’s long-term effectiveness and safety. Psychedelics cause profound experiences and even with their growing scientific interest and social acceptance, they must be approached with caution, he said.
“They’re powerful substances. They’re powerful medicine, so one has to handle them with a great deal of care,” Koch said.
Leila Okahata, Allen Institute for Brain Science

