More than half of all women in the United States are overweight or obese when they become pregnant. While being or becoming overweight during pregnancy can have potential health risks for moms, there are also hints that it may tip the scales for their kids to develop psychiatric disorders like autism or depression, which often affects one gender more than the other.
What hasn’t been understood however is how the accumulation of fat tissue in moms might signal through the placenta in a sex-specific way and rearrange the developing offspring’s brain.
To fill this gap, Duke postdoctoral researcher Alexis Ceasrine, Ph.D., and her team in the lab of Duke psychology & neuroscience professor Staci Bilbo, Ph.D., studied pregnant mice on a high-fat diet. In findings appearing November 28 in the journal Nature Metabolism, they found that moms’ high-fat diet triggers immune cells in the developing brains of male but not female mouse pups to overconsume the mood-influencing brain chemical serotonin, leading to depressed-like behavior.
The researchers said a similar thing may be happening in humans, too.
People with mood disorders like depression often lose interest in pleasurable activities. For mice, one innately pleasurable activity is drinking sugar water. Since mice preferentially sip sugar water over plain tap when given the choice, Ceasrine measured their drink preference as an estimate for depression. Males, but not females, born by moms on a high-fat diet lacked a preference for simple syrup over tap water. This rodent-like depression suggested to Ceasrine that moms’ nutrition while pregnant must have changed their male offspring’s brain during development.
One immediate suspect was serotonin. Often called the “happy” chemical, serotonin is a molecular brain messenger that’s typically reduced in people with depression.
Ceasrine and her team found that depressed-like male mice from high-fat diet moms had less serotonin in their brain both in the womb and as adults, suggesting these early impacts have lifelong consequences. Supplementing moms’ high-fat rodent chow with tryptophan, the chemical precursor to serotonin, restored males’ preference for sugar water and brain serotonin levels. Still, it was unclear how fat accumulation in mom lowered serotonin in their offspring.
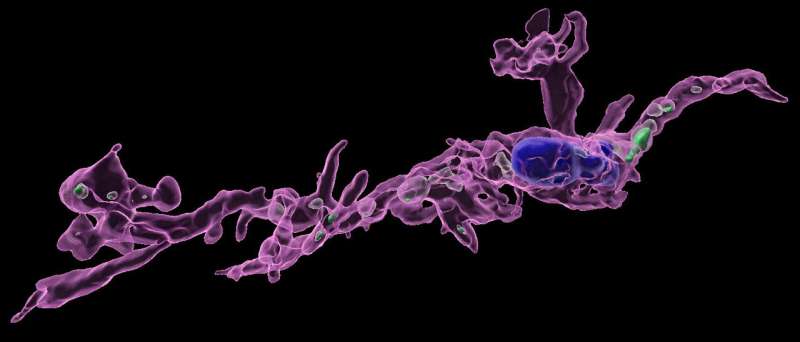
To get at this, the team investigated the resident immune cells of the brain: microglia.
Microglia are the understudied Swiss Army knives of the brain. Their jobs include serving as a security monitor for pathogens as well as a hearse to haul away dead nerve cells. Microglia also have ample space and appetites to consume healthy brain cells whole.
To see if microglia were overindulging in serotonin, Ceasrine analyzed the contents of their cellular “stomach,” the phagosome, with 3D imaging, and found that males born by moms on high-fat diets had microglia packed with more serotonin than those born to moms on a typical diet. This indicated that elevated fat accumulation during pregnancy somehow signals through the male but not female placenta to microglia and instructs them to overeat serotonin cells. How fat can signal through the placental barrier remained a mystery, though.
One thought was that bacteria were to blame.
“There’s a lot of evidence that when you eat a high fat diet, you actually end up with endotoxemia,” Ceasrine said. “It basically means that you have an increase in circulating bacteria in your blood, or endotoxins, which are just parts of bacteria.”
To test if endotoxins could be the critical messenger from mom to enwombed males, the team measured their presence and found that, indeed, high-fat diets during pregnancy beefed up endotoxin levels in the placenta and their offspring’s developing brain. Ceasrine said this may explain how fat accumulation triggers an immune response from microglia by increasing the presence of bacteria, resulting in overconsumed brain cells in male mice.
To see whether this may be true of humans as well, Ceasrine teamed up with Susan Murphy, Ph.D., a Duke School of Medicine associate professor in obstetrics and gynecology, who provided placental and fetal brain tissue from a previous study. Just as the researchers observed in mice, they found that the more fat measured in human placental tissue, the less serotonin was detected in the brains of males but not females.
Bilbo and Ceasrine are now starting to work out how and why female offspring are impacted differently when mom amasses high levels of fat during pregnancy. Fat doesn’t lead to depression in female mice, but it does make them less social, perhaps due to an overconsumption of the pro-social hormone oxytocin, instead of serotonin.
For now, this research highlights that not all placentas are created equally. This work may one day help guide clinicians and parents in better understanding and possible treatment or prevention of the origins of some mood disorders by considering early environmental factors, like fat accumulation during gestation.
So, why would the placenta treat male and female fetuses differently? Ceasrine was initially stumped when a student asked a similar question after a talk she gave to Bilbo’s class. Bilbo laughed and reiterated the question. But now they think they have it figured out.
“I was hugely pregnant at the time, and I was like, ‘Oh, wait. Pregnancy!'” Ceasrine recalled. “Men never have to carry a fetus, so they never have to worry about the kind of immune response of self versus non-self that you have to do when you’re a woman and you carry a baby.”
Duke University

