Northwestern Medicine scientists have identified a new therapeutic target for tumors and developed a compound which slowed tumor growth and bolsters immune responses in mice, according to research published in Science Advances.
The study authors first characterized the regulatory T-cells (Treg)—which act to prevent autoimmune responses—inside different types of cancerous tumors in human cancer samples and mouse models. While normal Treg cells work to temper autoimmune reactions like inflammation, these cells begin to behave differently once inside a tumor, effectively working to block the body’s anti-cancer efforts.
This phenomenon, called Treg fitness, has not been well understood until now, said Deyu Fang, Ph.D., the Hosmer Allen Johnson Professor of Pathology and senior author of the study.
“Tumor cells are smart. They take the immuno-suppressive Treg and use it to suppress anti-tumor responses,” said Fang, a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. “Even more so, the tumor makes these immuno-suppressive cells inside the tumor microenvironment even more suppressive than cells would be in normal populations, for example in the lymph nodes. So, the first question is: ‘Why does Treg become more suppressive?’ This paper provides the first answer.”
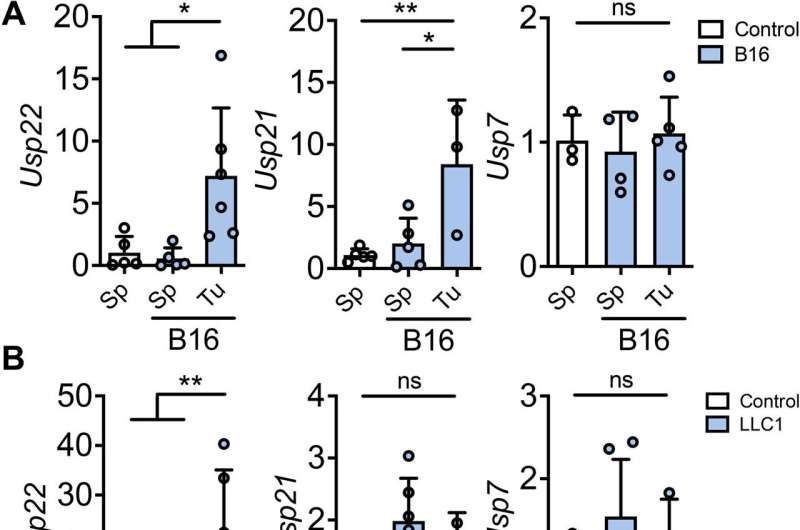
Investigators found that Treg cells inside tumors had higher levels of the protein FOXP3, which is known as a master regulator of the pathways involved in Treg development and function.
To understand why these cells produced more FOXP3, scientists increased levels of transforming growth factor–beta, a substance thought to play a role in Treg stability. According to the study, this resulted in increased levels of USP21 and USP22, proteins that protect FOXP3 from ubiquitin-mediated degradation in the cell.
Because targeting FOXP3 could result in an autoimmune response, scientists deleted USP21 and USP22 inside Treg cells in mice and found tumor growth slowed as a result.
“This study defines the first molecular pathway controlling Treg fitness,” Fang said. “We’ve also identified USP22 as an ideal therapeutic target.”
Investigators then sought to identify an inhibitor for USP22, which appeared to have the largest effect on tumor proliferation. Utilizing computer-aided drug design, the study authors identified a compound that slowed tumor growth and increased tumor rejection in mouse models without killing healthy cells.
With the compound now patented by Exomira Medicine, a start-up he co-founded with Huiping Liu, MD, Ph.D., associate professor of Pharmacology and Medicine in the division of Hematology and Oncology, Fang said he hopes to move to clinical trials in the future.
“Since our lab has discovered that USP22 also functions as an oncogene in cancer cells, this compound acts as chemotherapy and immunotherapy. It does both, which is why it can achieve this level of efficacy we see in slowing tumor growth,” Fang said. “We’re looking to move forward with the small molecule inhibitor we have identified, which is potentially translatable for cancer therapy.”
Olivia Dimmer, Northwestern University

