Bioengineered artificial skin has become an increasingly important and reliable platform for researchers to test the safety and efficacy of drugs and cosmetics. It can be produced on a large scale and is a good substitute for animal testing. The most promising technologies for production of in vitro models include 3D bioprinting.
Because it is recent, however, its performance has yet to be sufficiently validated in comparison to traditional manually produced models. This was the main aim of a study conducted by researchers at the University of São Paulo’s School of Pharmaceutical Sciences (FCF-USP) in Brazil. The results, reported in an article published in the journal Bioprinting, confirmed that the artificial skin achieved a similar performance.
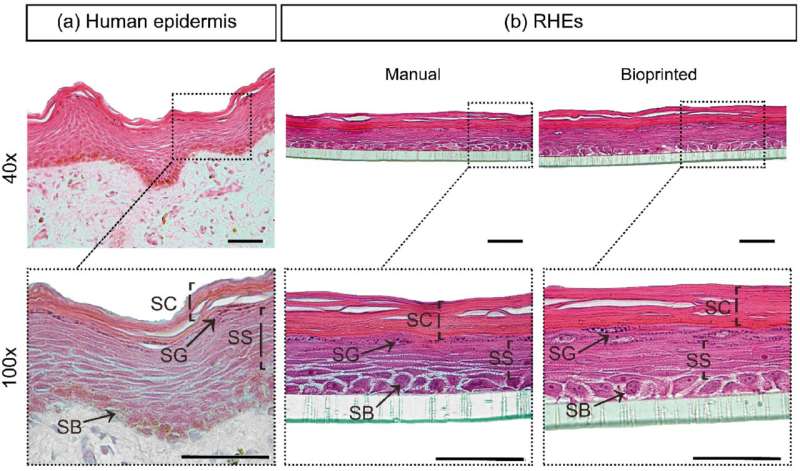
In the study, the researchers compared the conventional mimetic model based on manual pipetting with extrusion bioprinting, which “allows the in vitro reconstruction of a more relevant and representative model of human skin,” according to the authors of the article.
“Calling the model ‘artificial skin’ makes it sound synthetic, but actually it’s human tissue that closely resembles natural skin and is very suitable for safety and efficacy testing of bioactive compounds,” said Silvya Stuchi Maria-Engler, a professor and researcher at FCF-USP’s Department of Clinical and Toxicological Analysis.
Quality control and performance assessment standards established by international institutions such as the Organization for Economic Cooperation and Development (OECD) were used as validation criteria.
“The first was tissue morphology, which should be representative of human skin in vivo, with a stratified epidermis containing four layers: stratum basale, spinosum, granulosum and corneum. This means that in vitro reconstructed skin has the same functions as natural skin, which contains a selective barrier against the external medium for protection against chemical stressors [pollutants and topically applied products] and physical stressors [sunlight], while also retaining water,” said Denisse Esther Mallaupoma Camarena, co-first author of the article and a postdoctoral fellow at FCF-USP.
The next step was to assess the performance of the bioprinted skin as a barrier. Like natural skin, artificial skin should be able to prevent the penetration of detergents that cause irritation. To test this function, the researchers exposed the model to sodium dodecyl sulfate (SDS), a detergent that causes skin irritation, at different concentrations for 18 hours.
The last validation test entailed topical application of reference chemicals classified as irritants (such as acids, for example) or non-irritants (physiological solutions). The results showed the histology and cytoarchitecture of both in vitro reconstructed skin models to be consistent with internationally validated epidermic models. The quality of the bioprinted skin was as good as that of the manually reconstructed skin. Both responded equally well to irritants and distinguished between these and non-irritants.
“These findings prove that our bioprinted skin can be used instead of the Draize test, an acute toxicity test that applies the substance directly to rabbit skin. Besides the avoidance of animal testing, it’s less subject to human error and variability in the responses obtained by the cosmetics industry,” said Julia de Toledo Bagatin, first author of the article and a Ph.D. candidate at FCF-USP.
“Dissemination of part of the methods developed will help increase the use of alternatives to animal testing by the cosmetics industry, reinforcing our commitment to the cause,” said Juliana Lago, penultimate author of the article and scientific manager of Natura, a major Brazilian cosmetics company.
“Basic science done by academia produced the knowledge that served as a foundation for this project. The business-university partnership enabled us to accelerate application of this knowledge in the tissue reconstruction project and automation via bioprinting, all of which is important to our company.”
More reliable bioprinters
Although the main results of the study show that bioprinted skin can be used as a platform to test irritation in the laboratory, the researchers note the need for caution in using bioprinters. “They produce mimetic tissue by cell dispersion using a needle or conical nozzle, and depending on the system chosen, there may be cellular response alterations in the in vitro irritation test,” Maria-Engler said.
“Bioprinting is now being used in many fields, so it’s extremely important to acknowledge that the dispersion system chosen can damage the reliability of the tests by leading to altered responses, such as increased inflammation.”
The researchers plan to bioprint more complex models comprising epidermis, dermis and hypodermis with representative human skin cells. This will move the model closer to reality and produce more biologically relevant responses in safety and efficacy testing of products for topical use.
Julia Moióli, FAPESP

