Injections of an engineered antibody known as AMY109, given every month, were found to reduce lesion volume and lessen scar tissue and organ adhesions in monkeys with endometriosis.
Endometriosis occurs when cell tissues, usually found within the lining of the uterus, grow in areas outside the uterus. This tissue is hormonally sensitive and can become inflamed, especially during menstrual cycles, and can cause severe cramping, pain, and other symptoms depending on the area affected. These areas of endometrial-like tissue do not vacate during menstruation as cells within the lining usually do. Instead, they can form scar tissues, cysts, lesions, nodules, and connective tissues binding organs together. They also may make it harder for some to get pregnant.
Around 11% of women have endometriosis, with the highest rates among women in their 30s and 40s. There is no cure. Available treatments only target symptoms, with over-the-counter pain medicines and hormonal birth control, or in some cases, surgery to remove patches of endometriosis. A drug targeting the condition itself would be a huge benefit, especially for anyone planning to get pregnant.
According to the paper by researchers at Chugai Pharmaceutical, published in Science Translational Medicine, AMY109 may represent the first disease-modifying therapy for patients with endometriosis that does just that.
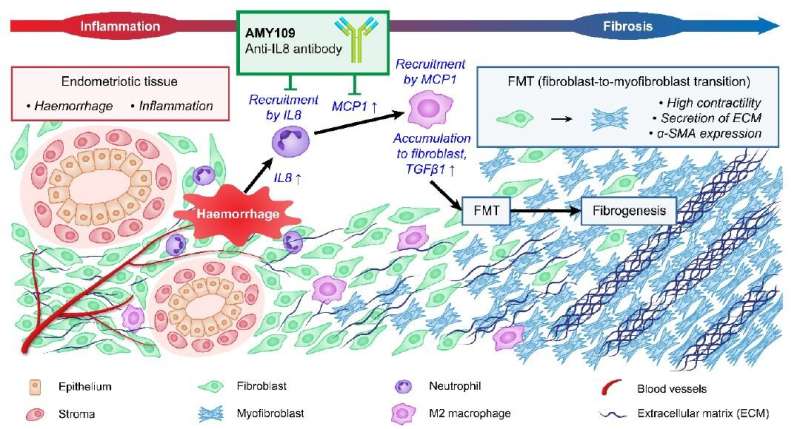
In previous research using human cells, the team had found high levels of the immune system signaling protein interleukin-8 associated with disease progression. While interleukin-8 is important for normal immune health by attracting white blood cells to an affected area, it also has been previously reported to induce the growth of endometriotic cells. So as healthy tissues getting invaded by rogue endometriotic cells respond by expressing interleukin-8, they could worsen the problem.
AMY109
The research team developed an antibody prototype to neutralize interleukin-8. After initial testing showed positive results, they further engineered the antibody with recycling antibody technology in which antibodies bind to antigens in a pH-dependent manner, allowing a single antibody molecule to target interleukin-8 multiple times. The addition of the recycling technology greatly increased the longevity of the treatment so that it only needs to be administered once per month. Their final formulation is called AMY109.
The researchers note that endometriosis is a chronic disease and that therapies should be highly potent and safe. With the current round of animal trials showing positive therapeutic outcomes, and a concurrent nonclinical safety assessment (also in monkeys) showing no adverse side effects, both look highly encouraging so far.
Justin Jackson , Medical Xpress

