Researchers develop liquid biopsy test for pediatric solid tumors
Pediatric solid tumors make up approximately 40% of all childhood cancers. While pediatric cancer is rare, children can develop a wide range of tumor types, located in different parts of the body, which can make the differential diagnosis challenging. Investigators at Children’s Hospital Los Angeles have developed a liquid biopsy for solid tumors that has the potential to aid in reaching a specific diagnosis when surgery or a tissue biopsy is not feasible. The study findings were published in the journal npj Precision Oncology.
“This is one of the first clinically validated liquid biopsy tests to be launched at a pediatric academic medical center,” says Jaclyn Biegel, Ph.D., Chief of Genomic Medicine and Director of the Center for Personalized Medicine at CHLA.
“We created a test that may be helpful in making a diagnosis, determining prognosis, and potentially identifying an effective therapy for children with solid tumors,” says Fariba Navid, MD, Medical Director of Clinical Research in the Cancer and Blood Disease Institute at CHLA. Dr. Navid and Dr. Biegel are co-senior authors of this study.
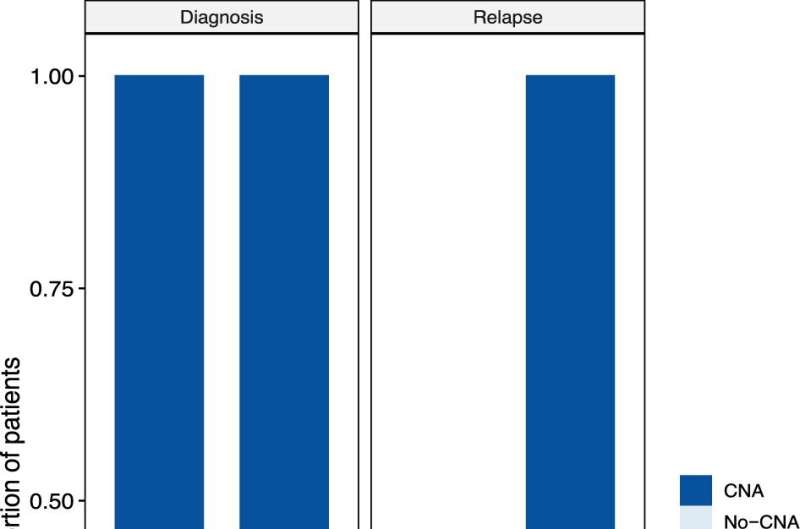
A specific test for pediatric tumors is required because the genetics of tumors that affect adults differ from those in children. Adult tumors tend to be caused by mutations—sequence-based changes in a gene— so most liquid biopsy tests have been developed specifically to identify these mutations. However, pediatric tumors arising from mutations are less common. In children, copy number changes—losing or having extra copies of one or more genes—or rearrangements of genes that result in gene fusions, are more characteristic.
For their research study, the CHLA team combined a technique known as Low-Pass Whole Genome Sequencing (LP-WGS) with targeted sequencing of cell-free DNA from plasma to detect copy number changes, as well as mutations and gene fusions, that are characteristic of pediatric solid tumors.
An important feature of the study was that it required a much smaller volume of sample than is required for liquid biopsy studies in adults. Since an infant or young child has a smaller blood volume, the assays needed to be scaled down to accommodate this difference.
To create the test, the researchers collaborated with clinical teams and research investigators at CHLA including Jesse Berry, MD, Director of Ocular Oncology and CHLA’s Retinoblastoma Program, as well as investigators involved in Oncology, Neurosurgery and Pathology and Laboratory Medicine. Leo Mascarenhas, MD, MS, Deputy Director of the Cancer and Blood Disease Institute at CHLA was also involved in the design and support of the project.
The first version of the test, launched in Nov. 2022, evaluates chromosomal copy number changes in blood samples, cerebrospinal fluid and the aqueous humor of the eye to aid in the clinical diagnosis for patients with solid tumors, brain tumors and retinoblastoma, respectively.
The next version of the clinical assay, available in about six months, will include detection of mutations and gene fusions.
The liquid biopsy-based genetic tests join the CHLA-developed OncoKids cancer panel, a next-generation sequencing-based assay designed to detect changes in DNA or RNA that are associated with pediatric leukemias, brain and solid tumors; the CHLA Cancer Predisposition Panel; RNAseq for cancer, a transcriptome-based assay using RNA sequencing; VMD4Kids, a panel for vascular and mosaic disorders; as well as methylation array-based profiling for pediatric brain tumors.
Children’s Hospital Los Angeles

