The biological function of the C-reactive protein, CRP, has long been unknown. Researchers at Linköping University in Sweden now show that this protein has a beneficial function in systemic lupus erythematosus, SLE, an inflammatory disease. But this is true only for one of CRP’s two forms, according to the study published in Journal of Autoimmunity.
Most of us have had a CRP blood test on more than one occasion. This is a very common routine health care test used to detect infection or systemic inflammation in the body. What is measured is the level of C-reactive protein, or CRP for short.
“CRP is an ancient protein and similar proteins can be found in all animals, even in primitive organisms. When a protein has been so well preserved throughout evolution, this usually means that it has an important function. CRP is used a lot in clinical care as a marker of ongoing inflammation, but few have studied its biological effects,” says Christopher Sjöwall, senior associate professor in the Department of Biomedical and Clinical Sciences, BKV, at Linköping University, LiU, and consultant at Reumatologiska kliniken (Rheumatology Clinic) in Östergötland.
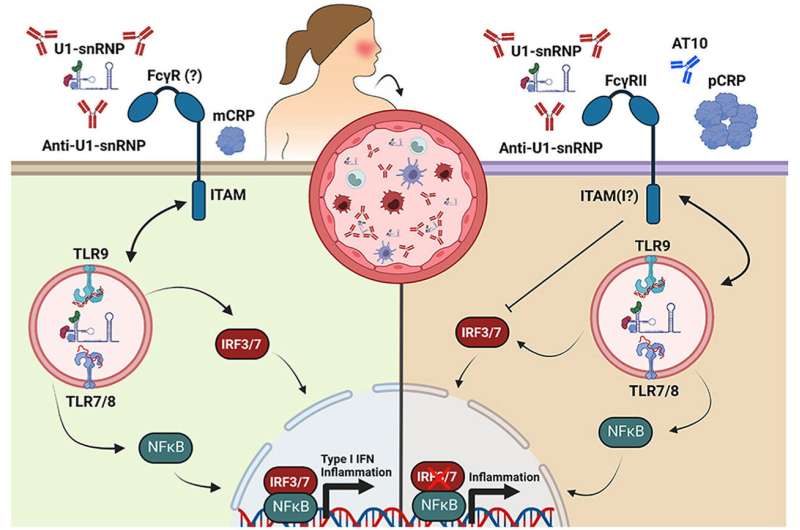
Christopher Sjöwall has spent many years researching the chronic inflammatory disease systemic lupus erythematosus, SLE. Somewhat simplified, this is a disease where the body’s immune system starts reacting to one’s own body, i.e., an autoimmune disease. One such reaction is the formation of antibodies, called autoantibodies, that target endogenous constituents. But in instances of SLE, when inflammatory activity in the body is high, SLE patients have a lower CRP level than could be expected. Previous studies on animals with lupus-prone disease have shown that adding CRP may result in a milder disease and lower autoantibody levels, which suggests that CRP could also have a beneficial function in human SLE, although the reason for this has not been clear.
One of the key players in SLE is a signaling protein called type I interferon. Interferon is normally a very important component in the body’s protection against infections. Interferon levels increase rapidly in the first hours following infection and slow it down by, for instance, making it more difficult for viruses to breed. Interferon levels should fall after a while. But in some diseases, such as SLE, the interferon remains in the system too long and causes damage.
“We have shown a mechanism that is likely quite important, where the relative lack of CRP in SLE patients with high disease activity leads to high interferon activity,” says Christopher Sjöwall.
In their study, the researchers examined the impact on interferon and other signaling proteins caused by CRP and SLE specific immune complexes formed by autoantibodies having reacted with material from dead cells. One thing they studied was what happened when serum from SLE patients with varying levels of CRP was added to healthy cells. They found that there was more interferon when CRP levels were low compared to when they were high, which they interpret as CRP contributing to reducing interferon response.
It also turned out that the shape of CRP is important, something that the Linköping researchers were the first to show. The protein consists of five identical units (pentamer shape), which can split and function independently (monomer shape). And the researchers found that it is only the pentamer shape CRP that reduces interferon activity.
“The finding that the pentamer shape of CRP can suppress the immune response is interesting also in the context of other diseases, such as various virus diseases,” says Marie Larsson, professor of virology in BKV at LiU and one of the other researchers behind the study.
These findings open up for research into medicines to reduce immune complexes and elevated interferon levels. But as interferon plays a key role in the body’s defense against infections, the treatment of SLE and similar diseases is a constant balancing act and further research is needed to find optimal treatment strategies.
Karin Söderlund Leifler, Linköping University

