Although chimeric antigen receptor (CAR) T cell therapy has shown potential in the treatment of hematological malignancies, its application in solid tumors is unsatisfactory due to the harsh physical barriers and immunosuppressive microenvironment.
The ideal CAR T therapy requires a novel armored CAR T cell engineered to navigate in the circulatory system, penetrate tumor tissues, and survive in the harsh tumor microenvironment to exert adequate immune effects.
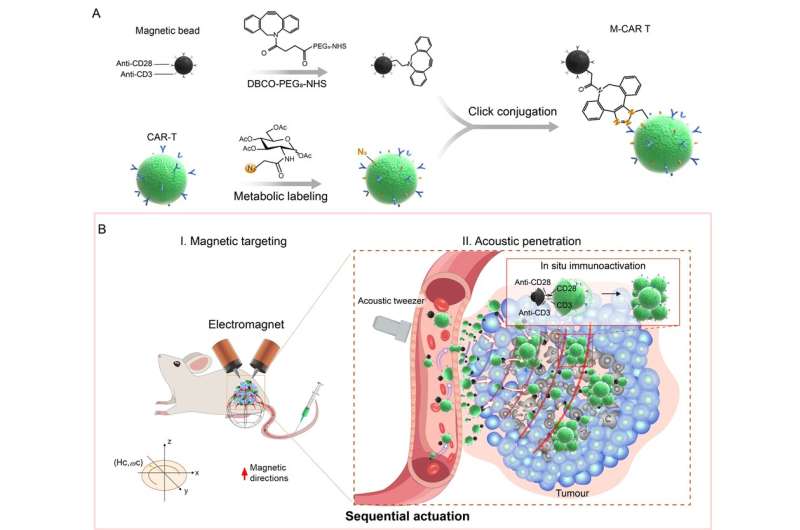
Recently, a research team led by Prof. Cai Lintao from the Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences has developed a CAR T cell-based microrobot (M-CAR T) with magnetic-acoustic sequential actuation that can autonomously navigate to tumor sites for cell immunotherapy by decorating CAR T with immunomagnetic beads using click conjugation.
The study was published in Advanced Materials on Feb. 17.
Based on immunomagnetic bead engineering, the M-CAR T demonstrated controllable anti-flow and obstacle avoidance movement and maintained an on-demand route under magnetic guidance. Meanwhile, it exhibited distinctive, acoustic manipulative properties whereby it could exert CAR T cell control and could actively penetrate artificial tumor tissues under magnetic-acoustic sequential actuation.
“Sequential actuation endows M-CAR Ts with magnetically actuated anti-flow and obstacle avoidance capabilities as well as acoustically actuated tumor tissue penetration, which enables efficient migration and accumulation in artificial tumor models,” said Prof. Cai.
In animal models, M-CAR Ts with sequential actuation achieved long-distance targeting and accumulated at the peritumoral area under programmable magnetic guidance. Subsequently, acoustic tweezers actuated M-CAR Ts to migrate into deep tumor tissues, resulting in a 6.6-fold increase in accumulated exogenous CD8+ CAR T cells compared with those with no actuation.
“Ingeniously, anti-CD3/CD28 immunomagnetic beads stimulate infiltrated CAR T proliferation and activation in situ, significantly enhancing their antitumor immune efficacies,” said Dr. Pan Hong, co-corresponding author of the study.
The M-CAR T maintains the bioactive properties of CAR T cells and is capable of magnetically propelled spatial targeting and acoustically actuated tumor penetration to cope with vascular anti-flow and obstacles to migrating into deep tumor. After entering tumor tissues, immunomagnetic beads in situ stimulated CAR T cells to overcome immunosuppressive tumor environments through efficient expansion and activation.
“Such sequential actuation-guided cell microrobots combine the merits of autonomous targeting and penetration of intelligent robots with in-situ immunoactivation of T cells. It holds considerable promise for clinical precision immunotherapies of solid cancer,” said Dr. Pan.
Li Yuan, Chinese Academy of Sciences

